Statement for the Record
- There is a tremendous need to develop new and effective medications for drug addiction and to increase access to treatment. This is articulated by a number of sources including the Institute of Medicine and a 1997 Consensus Development Conference on the Effective Medical Treatment of Heroin Addiction. Science is providing us with unprecedented opportunities to bridge the treatment gap.
- Research supported by the National Institute on Drug Abuse (NIDA) demonstrates that chronic drug use changes the brain in fundamental ways that persist long after the individual has stopped taking drugs. A PET image by a NIDA grantee demonstrates the lasting effects of drugs on the brain. Medications are needed to reverse the biological and behavioral components of drug addiction.
- Industry is reluctant to become involved in the development of anti addiction medications for a number of reasons, including financial reasons and for fear of being stigmatized by consumers. To stimulate the development of medications, Congress had authorized a drug discovery and development program within NIDA.
- According to the Pharmaceutical Research and Manufacturers Association it costs between $350 -$600 million to bring a new drug to market. It generally requires at least a ten-year commitment from the company with only one in ten candidate medications eventually being brought to market. Market incentives need to be provided to pharmaceutical companies to stimulate their interest in developing and bringing anti-addiction medications to market.
- Even after medications are developed, there are numerous obstacles that need to be overcome before the medications can be administered to patients. Existing federal, state and local restrictive policies and regulations can impede the availability of an approved anti-addiction medication. A case in point is LAAM, a medication for heroin addiction, that was very slow to become a medication available for treating heroin addiction.
- Medications such as buprenorphine and buprenorphine combined with naloxone have been found to be safe and efficacious as a treatment for opiate dependence in the studies done to date. In research settings, buprenorphine products have been found to be medications that will be well tolerated by addicts and have low value and desirability for sale on the street. These factors make this medication a prime candidate to be administered in less traditional environments, thus expanding access to populations who do not have access to methadone programs or are unsuited to them, such as adolescents. Buprenorphine products would not replace methadone.
- These buprenorphine products are not yet approved for this purpose by the Food and Drug Administration. The current regulations (21 CFR 291) for administration and delivery of narcotic medications in the treatment of narcotic dependent persons were written for the use of full agonist medications such as methadone with demonstrated abuse potential and do not take into account the unique pharmacological properties of these drugs. Buprenorphine products should increase the amount of treatment capacity available and expand the range of treatment options that can be used by physicians.
Mr. Chairman and Members of the Subcommittee, I am pleased to be here today to discuss how science is providing us with unprecedented opportunities to develop more and improved treatments for those suffering from the disease of addiction. The science I present this morning will be particularly relevant to the Drug Addiction Treatment legislation that is the topic of this hearing.
Given that drug abuse and addiction affects every American either directly or indirectly with the economic burden alone for drug abuse estimated to exceed $109 billion in the latest estimate, it is imperative that we utilize science to develop new and improved approaches to alleviate the devastating effects of this disease.
Scientific advances, particularly over the past decade, have catapulted both our understanding and our approaches to addiction. Research has in fact come to define addiction as a chronic, and for many people reoccurring disease characterized by compulsive drug seeking and use that results from the prolonged effects of drugs on the brain. A variety of studies in both humans and animals have demonstrated that chronic drug use does in fact change the brain in fundamental ways that persist long after the individual has stopped taking drugs. By using advanced brain imaging technologies we can see what we believe to be the biological core of addiction.
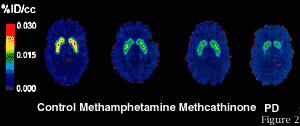 Figure 1 - What you are seeing in this image is dopamine transporter binding in four different adults. Brighter colors reflect greater dopamine binding capacity. The scan on the left is that of a non-drug user, the next is of a chronic methamphetamine user who was drug free for about three years when this image was taken, followed by a chronic methcathinone abuser who was also drug free for about three years. The last image is of the brain of an individual newly diagnosed with Parkinson's Disease. When compared with the control on the left, one can see the significant loss in the brain's ability to transport dopamine back into brain cells. Dopamine function is critical to emotional regulation, is involved in the normal experience of pleasure and is involved in controlling an individual's motor function. The data now suggest that every drug of abuse --be it nicotine, heroin, cocaine, marijuana or amphetamine--appears to increase the levels of the neurotransmitter dopamine in the brain pathways that control pleasure. It is this change in dopamine that we have come to believe is one fundamental characteristic of all addictions and is at the biological core of addiction.
Figure 1 - What you are seeing in this image is dopamine transporter binding in four different adults. Brighter colors reflect greater dopamine binding capacity. The scan on the left is that of a non-drug user, the next is of a chronic methamphetamine user who was drug free for about three years when this image was taken, followed by a chronic methcathinone abuser who was also drug free for about three years. The last image is of the brain of an individual newly diagnosed with Parkinson's Disease. When compared with the control on the left, one can see the significant loss in the brain's ability to transport dopamine back into brain cells. Dopamine function is critical to emotional regulation, is involved in the normal experience of pleasure and is involved in controlling an individual's motor function. The data now suggest that every drug of abuse --be it nicotine, heroin, cocaine, marijuana or amphetamine--appears to increase the levels of the neurotransmitter dopamine in the brain pathways that control pleasure. It is this change in dopamine that we have come to believe is one fundamental characteristic of all addictions and is at the biological core of addiction.This kind of fundamental knowledge which is generated by NIDA-supported researchers is used by our Medications Development Program staff to develop new targets and approaches for medications. For example, as reported just last week in the Journal Nature, NIDA-supported scientists in collaboration with researchers from the Institut National De La Sante Et De La Recherche Medicale in France applied basic research findings about dopamine and cocaine addiction to develop and test a potential new compound for cocaine addiction. The researchers have developed a compound, BP 897, that appears to reduce cocaine "craving" in animals. The selective partial agonist BP 897 works on the D3 receptor, one of the known dopamine receptors found on the surface of brain cells, that is thought to be involved in the reinforcing effects of cocaine. The compound was found to reduce cocaine-seeking behavior in rats trained to associate cocaine taking with a light. The light represents an environmental cue or stimulus, which the rat learns to associate with cocaine administration, the same way that the mere sight of drug paraphernalia can trigger craving in human cocaine addicts who have been abstinent for years. The compound was found to block the cued response, thus bringing us one step closer to having a medication for cocaine addiction.
Given that the development of new and effective medications for the treatment of addiction is both a national need and a NIDA priority, it is imperative that we capitalize on recent research advances like this to rapidly bring new medications to the clinical toolboxes of front line clinicians who are treating addiction. Just as medications have been developed for other chronic diseases, such as hypertension, diabetes, and cancer, drug addiction is a disease that also merits medication for its treatment.
We have already made great progress in bringing quite an array of useful tools to drug abuse professionals to treat addicted individuals, such as the readily available nicotine addiction therapies; the most effective medications to date for heroin addiction, methadone and LAAM, levo-alpha-acetyl-methadol (trademark ORLAAM), which I will discuss in more detail shortly; as well as a number of notable standardized behavioral interventions, such as cognitive behavioral therapies and contingency management, that are effective in treating both adults and adolescents.
Although we are optimistic that science can help bring more effective treatments to the Nation's forefront, there is a tremendous national need to bring these medications to fruition at a quicker pace and to increase access to treatment. When one takes into account that there are approximately 810,000 persons in need of treatment for heroin addiction and only space in the existing methadone clinic system for 180,000 patients, the need for expanding treatment for this population takes on new light.
The need to bridge the treatment gap by developing new treatments that are accessible to those in need is a point articulated strongly by the Institute of Medicine, as well as the National Institutes of Health's November 1997 Consensus Development Conference on the Effective Medical Treatment of Heroin Addiction, among others. Methadone, the medication that has been shown to have the most favorable impact on mortality, morbidity and the interruption of drug-seeking behaviors is still inaccessible to many. An abundance of studies have also consistently shown that methadone is highly effective in retaining in treatment a large proportion of patients, reducing their intravenous drug use and criminal activity and enhancing their social productivity. Despite the clinical success of medications such as methadone, pharmacotherapy for the treatment of drug addiction has received far too little attention, particularly from the pharmaceutical industry.
In an effort to stimulate the availability of medications to treat drug addiction, Congress authorized (P.L. 99-570) a drug discovery and development program within NIDA. To work with industry to perform the research and development necessary to secure FDA marketing approval for new medications to treat drug addiction, NIDA established its own Medications Development Division (MDD) in 1990, which is headed by my colleague, Dr. Frank Vocci.
By funding research at every step of the complex medications development process; from the chemical syntheses, and the biochemical, behavioral, pharmacological, and toxicological testing, to the clinical trials that evaluate the safety and efficacy of potential medications, NIDA offers pharmaceutical firms and academia the resources and the technical assistance to perform the necessary next steps to bring a medication to market. MDD also aggressively pursues ways to facilitate and accelerate the medications development process. An additional economic incentive for anti-addiction medication development can come from the Food and Drug Administration under the Orphan Drug Act
It was NIDA's MDD that shepherded through the final development of the Nation's most recent tool to combat heroin addiction, LAAM. LAAM, like the better known medication methadone, stabilizes the brains of heroin addicts by ameliorating craving for heroin and preventing withdrawal symptoms. Treatment with methadone requires daily dosing, whereas LAAM is effective for up to three days.
LAAM was approved by the Food and Drug Administration (FDA) in 1993 after an extensive research cycle that began with the 1968 discovery that the analgesic LAAM might be a potential treatment for heroin addiction. Clinical research on LAAM continued intermittently throughout the 1970s, and 1980s. As LAAM was "off patent" for more than 20 years, no private sector entity attempted to develop LAAM when it was determined that additional clinical trials would be required. NIDA advertised and awarded a contract to a small U.S. company, Biometrics Research Institute, to develop and submit a New Drug Application for LAAM. This contract eventually resulted in the approval of LAAM by the FDA. Once the drug was approved in July 1993, there were still many barriers to overcome. For example, LAAM can only be distributed to clinics and hospitals that operate licensed narcotic treatment programs. Clinics had to receive approval to dispense LAAM from Federal, State and some local authorities. Also, to permit LAAM to be distributed to treatment programs, states first had to schedule or reschedule the medication into a Schedule II status, allowing use in existing treatment settings. States also had to develop treatment regulations that in some cases took over four years to receive the necessary approvals. Given these factors, it is not difficult to understand why, as of May 21, 1999 according to the FDA, of the 934 outpatient Narcotic Treatment Programs in the nation approved for maintenance and detoxification treatment only 376 are approved to dispense LAAM. We estimate that 5000 to 6000 patients are currently receiving LAAM.
This short case scenario of the financial and time consuming challenges that were overcome to bring LAAM to the market exemplifies why pharmaceutical companies do not want to invest in anti-addiction medications. There are virtually no market incentives for pharmaceutical companies to develop medications for drug addiction. In fact the congressionally mandated 1995 Institute of Medicine Report, "The Development of Medications for the Treatment of Opiate and Cocaine Addictions," states that the disincentives for the pharmaceutical industry to become involved in anti-addiction medications far outweigh the incentives. The cost of bringing any new drug to market is enormous, according to the Pharmaceutical Research and Manufacturers Association. It costs anywhere from $350 to $600 million, and generally requires 10 or more years of a company's investment, with only one in ten candidate medications eventually being brought to market. Not only are pharmaceutical companies hesitant to develop medications for market and financial reasons, but they are fearful of the stigma that society will attach to them or their drugs if they were to develop a compound for drug addiction, especially if the compound has other medical uses. For example, methadone was first developed as an analgesic. When it was approved for treatment of opiate addiction it became subject to additional layers of regulation and became stigmatized. As a result, sales dramatically plummeted. The industry is well aware of the problems encountered by medications such as methadone and LAAM. They want an adequate return on their investment before deciding to enter a new area of drug development. Unfortunately the majority of the factors they look at, including the size of the market, the nature of third party reimbursement for drug abuse, the regulatory requirements for development and approval, the clinical trials that need to be conducted, and the regulatory barriers concerning how the product may be administered among others, are seen as problematic to the company. The bottom line is, if we want pharmaceutical companies to invest in anti-addiction medications, incentives must be provided to them to stimulate their interest.
For our part, NIDA will continue supporting basic research, as well as researching and developing the most effective treatments for the populations we are treating. We have learned a lot about treating opiate addicts from the methadone and LAAM experience. We have learned, for example that medications that are full agonists such as methadone and LAAM are very effective in treating heroin addiction. They are also effective in helping to reduce the spread of infectious diseases, such as HIV, and hepatitis through reduction of injection practices. Research has also taught us that we need to develop medications that will be readily used and accepted by the populations we are treating. These are some of the reasons that we are working to bring other medications to the Nation's forefront - buprenorphine and buprenorphine combined with naloxone.
Buprenorphine is a partial mu opiate receptor agonist with unique properties which differentiate it from full agonists such as methadone or LAAM. Partial agonists exhibit ceiling effects, meaning increasing the dose only has effects to a certain level, so it will be well tolerated by addicts and have low value and desirability for sale on the street. Naloxone has been added to buprenorphine to minimize its abuse potential, particularly with respect to parenteral (intravenous) abuse. These factors make this medication a prime candidate to be administered in less traditional environments, thus potentially expanding treatment to populations who either do not have access to methadone programs or are unsuited to them, such as adolescents. Just next month we will be enrolling patients in a buprenorphine/naloxone office based treatment trial that will help us better refine and establish best practices for administering this medication.
Buprenorphine and buprenorphine/naloxone (bup/nx) would not be a replacement for methadone or LAAM, but yet another treatment option for both physicians and patients. Both methadone and LAAM would continue to remain an important part of the treatment continuum. Buprenorphine and bup/nx, however, could help reach new groups of opiate addicts, such as those in the seven states that do not currently have methadone programs; those who may be reluctant to enter a methadone program; and adolescents and new heroin addicts who are unsuited to methadone. As in all forms of medicine, it is critically important to allow physicians and patients to have access to as many forms of treatment as may be available, and to chose the best match for each individual.
It is also important that physicians are knowledgeable about addiction and the comprehensive approach required for treatment to be effective. As noted in the November 1997 National Institutes of Health Consensus Development Statement, non-pharmacological supportive services are pivotal to successful treatment. Ongoing substance abuse counseling and other psychosocial therapies, vocational rehabilitation, and other needed medical and social services are essential for program retention and positive outcome.
NIDA will continue to work with health care professionals and to conduct trials of buprenorphine products in varied environments, such as primary care and mental health clinics, to expand treatment availability and to ensure the effectiveness of these medications in real world settings. It should be noted, however, that neither of these products have yet been approved by the FDA to treat narcotic addiction.
As with all medical conditions, science will continue to provide us with the best hope. It is science that will help us develop even more novel approaches to treat addiction. Our only other hope is that when these new treatments are developed and tested, they will rapidly be provided to those who need them most.
Thank you for the opportunity to testify before this Subcommittee. My colleague and I will be happy to respond to any questions.
