Good Morning, Mr. Chairman and Members of the Subcommittee. Thank you for inviting the National Institute on Drug Abuse (NIDA), a component of the National Institutes of Health (NIH), to participate in this important hearing and contribute what I believe will be useful insights into the growing and intertwined problems of prescription pain relievers and heroin abuse in this country.
Background
The abuse of and addiction to opioids such as heroin, morphine, and prescription pain relievers is a serious global problem that affects the health, social, and economic welfare of all societies. It is estimated that between 26.4 million and 36 million people abuse opioids worldwide,[1] with an estimated 2.1 million people in the United States suffering from substance use disorders related to prescription opioid pain relievers in 2012 and an estimated 467,000 addicted to heroin.[2] The consequences of this abuse have been devastating and are on the rise. For example, the number of unintentional overdose deaths from prescription pain relievers has soared in the United States, more than quadrupling since 1999. There is also growing evidence to suggest a relationship between increased non-medical use of opioid analgesics and heroin abuse in the United States.[3]
To address the complex problem of prescription opioid and heroin abuse in this country, we must recognize and consider the special character of this phenomenon, for we are asked not only to confront the negative and growing impact of opioid abuse on health and mortality, but also to preserve the fundamental role played by prescription opioid pain relievers in healing and reducing human suffering. That is, scientific insight must strike the right balance between providing maximum relief from suffering while minimizing associated risks and adverse effects.
Abuse of Prescription Opioids: Scope and Impact
Prescription opioids are one of the three main broad categories of medications that present abuse liability, the other two being stimulants and central nervous system (CNS) depressants.
Several factors are likely to have contributed to the severity of the current prescription drug abuse problem. They include drastic increases in the number of prescriptions written and dispensed, greater social acceptability for using medications for different purposes, and aggressive marketing by pharmaceutical companies. These factors together have helped create the broad “environmental availability” of prescription medications in general and opioid analgesics in particular.
To illustrate this point, the total number of opioid pain relievers prescribed in the United States has skyrocketed in the past 25 years (Fig. 1).[4] The number of prescriptions for opioids(like hydrocodone and oxycodone products) have escalated from around 76 million in 1991 to nearly 207 million in 2013, with the United States their biggest consumer globally, accounting for almost 100 percent of the world total for hydrocodone (e.g., Vicodin) and 81 percent for oxycodone (e.g., Percocet).[5]
 Figure 1. Opioid Prescriptions Dispensed by US Retail Pharmacies. IMS Health, Vector One: National, Years 1991-1996, Data Extracted 201. IMS Health, National Prescription Audit, Years 1997-2013, Data Extracted 2014.
Figure 1. Opioid Prescriptions Dispensed by US Retail Pharmacies. IMS Health, Vector One: National, Years 1991-1996, Data Extracted 201. IMS Health, National Prescription Audit, Years 1997-2013, Data Extracted 2014.This greater availability of opioid (and other) prescribed drugs has been accompanied by alarming increases in the negative consequences related to their abuse.[6] For example, the estimated number of emergency department visits involving nonmedical use of opioid analgesics increased from 144,600 in 2004 to 305,900 in 2008;[7] treatment admissions for primary abuse of opiates other than heroin increased from one percent of all admissions in 1997 to five percent in 2007[8]; and overdose deaths due to prescription opioid pain relievers have more than tripled in the past 20 years, escalating to 16,651 deaths in the United States in 2010.[9]
In terms of abuse and mortality, opioids account for the greatest proportion of the prescription drug abuse problem. Deaths related to prescription opioids began rising in the early part of the 21st century. By 2002, death certificates listed opioid analgesic poisoning as a cause of death more commonly than heroin or cocaine.[10]
Because prescription opioids are similar to, and act on the same brain systems affected by, heroin and morphine (Fig.2), they present an intrinsic abuse and addiction liability, particularly if they are used for non-medical purposes. They are most dangerous and addictive when taken via methods that increase their euphoric effects (the “high”), such as crushing pills and then snorting or injecting the powder, or combining the pills with alcohol or other drugs. Also, some people taking them for their intended purpose risk dangerous adverse reactions by not taking them exactly as prescribed (e.g., taking more pills at once, or taking them more frequently or combining them with medications for which they are not being properly controlled); and it is possible for a small number of people to become addicted even when they take them as prescribed, but the extent to which this happens currently is not known. It is estimated that more than 100 million people suffer from chronic pain in this country,[11] and for some of them, opioid therapy may be appropriate. The bulk of American patients who need relief from persistent, moderate-to-severe non-cancer pain have back pain conditions (approximately 38 million) or osteoarthritis (approximately 17 million).[12] Even if a small percentage of this group develops substance use disorders (a subset of those already vulnerable to developing tolerance and/or clinically manageable physical dependence[13]), a large number of people could be affected. Scientists debate the appropriateness of chronic opioid use for these conditions in light of the fact that long-term studies demonstrating that the benefits outweigh the risks have not been conducted. In June 2012, NIH and FDA held a joint meeting on this topic,[14] and now FDA is requiring companies who manufacture long-acting and extended-release opioid formulations to conduct post-marketing research on their safety.[15]
The Effects of Opioid Abuse on the Brain and Body. Opioids include drugs such as OxyContin and Vicodin that are mostly prescribed for the treatment of moderate to severe pain. They act by attaching to specific proteins called opioid receptors, which are found on nerve cells in the brain, spinal cord, gastrointestinal tract, and other organs in the body. When these drugs attach to their receptors, they reduce the perception of pain and can produce a sense of well-being; however, they can also produce drowsiness, mental confusion, nausea, and constipation.[16] The effects of opioids are typically mediated by specific subtypes of opioid receptors (mu, delta, and kappa) that are activated by the body’s own (endogenous) opioid chemicals (endorphins, encephalins). With repeated administration of opioid drugs (prescription or heroin), the production of endogenous opioids is inhibited, which accounts in part for the discomfort that ensues when the drugs are discontinued (i.e., withdrawal). Adaptations of the opioid receptors’ signaling mechanism have also been shown to contribute to withdrawal symptoms.
Opioid medications can produce a sense of well-being and pleasure because these drugs affect brain regions involved in reward. People who abuse opioids may seek to intensify their experience by taking the drug in ways other than those prescribed. For example, extended-release oxycodone is designed to release slowly and steadily into the bloodstream after being taken orally in a pill; this minimizes the euphoric effects. People who abuse pills may crush them to snort or inject which not only increases the euphoria but also increases the risk for serious medical complications, such as respiratory arrest, coma, and addiction. When people tamper with long-acting or extended-release medicines, which typically contain higher doses because they are intended for release over long periods, the results can be particularly dangerous, as all of the medicine can be released at one time. Tampering with extended release and using by nasal, smoked, or intravenous routes produces risk both from the higher dose and from the quicker onset.
Opioid pain relievers are sometimes diverted for nonmedical use by patients or their friends, or sold in the street. In 2012, over five percent of the U.S. population aged 12 years or older used opioid pain relievers non-medically.[17] The public health consequences of opioid pain reliever abuse are broad and disturbing. For example, abuse of prescription pain relievers by pregnant women can result in a number of problems in newborns, referred to as neonatal abstinence syndrome (NAS), which increased by almost 300 percent in the United States between 2000 and 2009.[18] This increase is driven in part by the high rate of opioid prescriptions being given to pregnant women. In the United States, an estimated 14.4 percent of pregnant women are prescribed an opioid during their pregnancy.[19]
Prescription opioid abuse is not only costly in economic terms (it has been estimated that the nonmedical use of opioid pain relievers costs insurance companies up to $72.5 billion annually in health-care costs[20]) but may also be partly responsible for the steady upward trend in poisoning mortality. In 2010, there were 13,652 unintentional deaths from opioid pain reliever (82.8 percent of the 16,490 unintentional deaths from all prescription drugs),[21] and there was a five-fold increase in treatment admissions for prescription pain relievers between 2001 and 2011 (from 35,648 to 180,708, respectively).[22] In the same decade, there was a tripling of the prevalence of positive opioid tests among drivers who died within one hour of a crash.[23]
A property of opioid drugs is their tendency, when used repeatedly over time, to induce tolerance. Tolerance occurs when the person no longer responds to the drug as strongly as he or she did at first, thus necessitating a higher dose to achieve the same effect. The establishment of tolerance hinges on the ability of abused opioids (e.g., OxyContin, morphine) to desensitize the brain’s own natural opioid system, making it less responsive over time.[24] This tolerance contributes to the high risk of overdose during a relapse to opioid use after a period in recovery; users who do not realize they may have lost their tolerance during a period of abstinence may initially take the high dosage that they previously had used before quitting, a dosage that produces an overdose in the person who no longer has tolerance.[25] Another contributing factor to the risk of opioid-related morbidity and mortality is the combined use of benzodiazepines (BZDs) and/or other CNS depressants, even if these agents are used appropriately. Thus, patients with chronic pain who use opioid analgesics along with BZDs (and/or alcohol) are at higher risk for overdose. Unfortunately, there are few available practice guidelines for the combined use of CNS depressants and opioid analgesics; such cases warrant much closer scrutiny and monitoring.[26] Finally, it must be noted in this context that, although more men die from drug overdoses than women, the percentage increase in deaths seen since 1999 is greater among women: Deaths from opioid pain relievers increased five-fold between 1999 and 2010 for women versus 3.6 times among men.[27]
Relationship between Prescription Opioids and Heroin Abuse
The recent trend of a switch from prescription opioids to heroin seen in some communities in our country alerts us to the complex issues surrounding opioid addiction and the intrinsic difficulties in addressing it through any single measure such as enhanced diversion control (Fig.3). Of particular concern has been the rise in new populations of heroin users, particularly young people.
 Figure 3. Growing evidence suggests that abusers of prescription opioids are shifting to heroin as prescription drugs become less available or harder to abuse. For example, a recent increase in heroin use accompanied a downward trend in Oxycontin abuse following the introduction of an abuse-deterrent formulation of that medication (dashed vertical line).3
Figure 3. Growing evidence suggests that abusers of prescription opioids are shifting to heroin as prescription drugs become less available or harder to abuse. For example, a recent increase in heroin use accompanied a downward trend in Oxycontin abuse following the introduction of an abuse-deterrent formulation of that medication (dashed vertical line).3The emergence of chemical tolerance toward prescribed opioids, perhaps combined in a smaller number of cases with an increasing difficulty in obtaining these medications illegally[28], may in some instances explain the transition to abuse of heroin, which is cheaper and in some communities easier to obtain than prescription opioids.
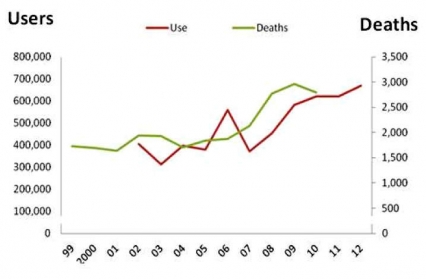
The number of past-year heroin users in the United States nearly doubled between 2005 and 2012, from 380,000 to 670,000 (Fig. 4).[29] Heroin abuse, like prescription opioid abuse, is dangerous both because of the drug’s addictiveness and because of the high risk for overdosing. In the case of heroin, this danger is compounded by the lack of control over the purity of the drug injected and its possible contamination with other drugs (such as fentanyl, a very potent prescription opioid that is also abused by itself).[30] All of these factors increase the risk for overdosing, since the user can never be sure of the amount of the active drug(or drugs) being taken. In 2010, there were 2,789 fatal heroin overdoses, approximately a 50 percent increase over the relatively constant level seen during the early 2000s.[31] What was once almost exclusively an urban problem is spreading to small towns and suburbs. In addition, the abuse of an opioid like heroin, which is typically injected intravenously, is also linked to the transmission of human immunodeficiency virus (HIV), hepatitis (especially Hepatitis C), sexually-transmitted infections, and other blood-borne diseases, mostly through the sharing of contaminated drug paraphernalia but also through the risky sexual behavior that drug abuse may engender.
NIDA Activities to Stem the Tide of Prescription Opioid and Heroin Abuse
NIDA first launched its prescription drug abuse public health initiative in 2001. Our evidence-based strategy calls for a comprehensive three-pronged approach consisting of (1) enhancing our understanding of pain and its management; (2) preventing overdose deaths; and (3) effectively treating opioid addiction.
Research on Pain and Next Generation Analgesics. Although opioid medications effectively treat acute pain and help relieve chronic pain for some patients,[32] their addiction risk presents a dilemma for healthcare providers who seek to relieve suffering while preventing drug abuse and addiction. Little is yet known about the risk for addiction among those being treated for chronic pain or about how basic pain mechanisms interact with prescription opioids to influence addiction potential. To better understand this, NIDA launched a research initiative on "Prescription Opioid Use and Abuse in the Treatment of Pain." This initiative encourages a multidisciplinary approach using both human and animal studies to examine factors (including pain itself) that predispose or protect against opioid abuse and addiction. Funded grants cover clinical neurobiology, genetics, molecular biology, prevention, treatment, and services research. This type of information will help develop screening and diagnostic tools that physicians can use to assess the potential for prescription drug abuse in their patients. Because opioid medications are prescribed for all ages and populations, NIDA is also encouraging research that assesses the effects of prescription opioid abuse by pregnant women, children, and adolescents, and how such abuse in these vulnerable populations might increase the lifetime risk of substance abuse and addiction.
Another important initiative pertains to the development of new approaches to treat pain. This includes research to identify new pain relievers with reduced abuse, tolerance, and dependence risk, as well as devising alternative delivery systems and formulations for existing drugs that minimize diversion and abuse (e.g., by preventing tampering and/or releasing the drug over a longer period of time) and reduce the risk of overdose deaths. New compounds are being developed that exhibit novel properties as a result of their combined activity on two different opioid receptors (i.e., mu and delta). Preclinical studies show that these compounds can induce strong analgesia but fail to produce tolerance or dependence. Researchers are also getting closer to developing a new generation of non–opioid-based medications for severe pain that would circumvent the brain reward pathways, thereby greatly reducing abuse potential. This includes compounds that work through a type of cannabinoid receptor found primarily in the peripheral nervous system. NIDA is also exploring the use of non-medication strategies for managing pain. An example is the use of “neurofeedback,” a novel modality of the general biofeedback approach, in which patients learn to regulate specific regions in their brains by getting feedback from real-time brain images. This technique has shown promising results for altering the perception of pain in healthy adults and chronic pain patients and could even evolve into a powerful psychotherapeutic intervention capable of rescuing the circuits and behaviors impaired by addiction.
Developing More Effective Means for Preventing Overdose Deaths. The opioid overdose antidote naloxone has reversed more than 10,000 overdose cases between 1996 and 2010, according to CDC. [33] For many years, naloxone was available only in an injectable formulation and was generally only carried by medical emergency personnel. However, FDA has recently approved a new hand-held auto-injector of naloxone to reverse opioid overdose that is specifically designed to be given by family members or caregivers. In order to expand the options for effectively and rapidly counteracting the effects of an overdose, NIDA is also supporting the development of a naloxone nasal spray—a needle-free, unit-dose, ready-to-use opioid overdose antidote that can easily be used by an overdose victim, a companion, or a wider range of first responders (e.g., police) in the event of an emergency.[34]
Research on the Treatment of Opioid Addiction. Drug abuse treatment must address the brain changes mentioned earlier, both in the short and long term. When people addicted to opioids first quit, they undergo withdrawal symptoms, which may be severe (pain, diarrhea, nausea, vomiting, hypertension, tachycardia, seizures). Medications can be helpful in this detoxification stage, easing craving and other physical symptoms that can often trigger a relapse episode. However, this is just the first step in treatment. Medications have also become an essential component of an ongoing treatment plan, enabling opioid-addicted persons to regain control of their health and their lives.
Agonist medications developed to treat opioid addiction work through the same receptors as the addictive drug but are safer and less likely to produce the harmful behaviors that characterize addiction, because the rate at which they enter and leave the brain is slower. The three classes that have been developed to date include (1) agonists, e.g., methadone (Dolophine or Methadose), which activate opioid receptors; (2) partial agonists, e.g., buprenorphine (Subutex, Suboxone), which also activate opioid receptors but produce a diminished response; and (3) antagonists, e.g., naltrexone (Depade, Revia, Vivitrol), which block the receptor and interfere with the rewarding effects of opioids. Physicians can select from these options on the basis of a patient’s specific medical needs and other factors. Research has shown methadone- and buprenorphine-containing medicines, when administered in the context of an addiction treatment program, can effectively maintain abstinence from other opioids and reduce harmful behaviors; we believe their gradual onset and long duration contribute to this ability to “stabilize” patient behavior.
Scientific research has established that medication-assisted treatment of opioid addiction is associated with decreases in the number of overdoses from heroin abuse,[35] increases retention of patients in treatment and decreases drug use, infectious disease transmission, and criminal activity. For example, studies among criminal offenders, many of whom enter the prison system with drug abuse problems, showed that methadone treatment begun in prison and continued in the community upon release extended the time parolees remained in treatment, reduced further drug use, and produced a three-fold reduction in criminal activity (Fig. 5). Investment in medication-assisted treatment of opioid addiction also makes good economic sense. According to a 2005 published analysis that tracked methadone patients from age 18 to 60 and included such variables as heroin use, treatment for heroin use, criminal behavior, employment, and healthcare utilization, every dollar spent on methadone treatment yields $38 in related economic benefits—seven times more than previously thought.[36]
 Figure 5. Methaone Treatment Pre-and Post Release Increases Treatment Retention and Reduces drug use
Figure 5. Methaone Treatment Pre-and Post Release Increases Treatment Retention and Reduces drug use(Findings at 12 months Post-Release)
Buprenorphine is worth highlighting in this context for its pioneering contributions to addiction treatment. NIDA-supported basic and clinical research led to the development of this compound, which rigorous studies have shown to be effective, either alone or in combination with naloxone, in significantly reducing opiate drug abuse and cravings.
The arrival of buprenorphine represented a significant health services delivery innovation. FDA approved Subutex® (buprenorphine) and Suboxone® tablets (buprenorphine/naloxone formulation) in October 2002, making them the first medications to be eligible for prescribing under the Drug Addiction Treatment Act of 2000. Subutex contains only buprenorphine hydrochloride. This formulation was developed as the initial product. The second medication, Suboxone, contains naloxone to guard against misuse (by initiating withdrawal if the formulation is injected). Subutex and Suboxone are less tightly controlled than methadone because they have a lower potential for abuse and are less dangerous in an overdose. As patients progress in their therapy, their doctor may write a prescription for a take-home supply of the medication. To date, of the nearly 872,615 potential providers registered with the Drug Enforcement Administration (DEA), 25,021 registered physicians are authorized to prescribe these two medications. The development of buprenorphine and its authorized use in physicians' offices gives opioid-addicted patients more medical options and extends the reach of addiction medication to remote populations.
Medication-assisted treatments remain grossly underutilized in many addiction treatment settings, where stigma and negative attitudes (based on the misconception that buprenorphine or methadone “substitute a new addiction for an old one”) persist among clinic staff and administrators. This leads to insufficient dosing or limitations on the duration of use of these medications (when they are used at all), which often leads to treatment failure and the perception that the drugs are ineffective, further reinforcing the negative attitudes toward their use.[37] Policy and regulatory barriers also can present obstacles.
Integrating Drug Treatment into Healthcare Settings
Medication-assisted treatment will be most effective when offered within the larger context of a high-quality delivery system that addresses opioid addiction not only with medication but also with behavioral interventions to support treatment participation and progress, infectious disease identification and treatment (especially HIV and HCV), screening and treatment of co-morbid psychiatric diseases, and overdose protection (naloxone). NIDA’s research over the last two decades has provided us with evidence that a high quality treatment system to address opioid addiction must include all these components, yet there are currently very few systems in the United States that provide this bundle of effective services.[38] Health care reform—with a focus on both expanding access to treatment and improving the quality of care—offers hope that we may be better able to integrate drug treatment into healthcare settings and offer comprehensive treatment services for opioid addiction. We also are examining ways to use health care reform and the focus on health promotion and wellness to pay for and deliver prevention interventions targeted at children, adolescents, young adults, and high-risk adult populations like those with chronic pain or returning veterans.
Prevention, Education, and Outreach
 Figure 6. Medscape's Test-and-Teach is one example of NIDA's multiplatform approached to enhance a physician's ability to properly manage pain while preventing the abuse of prescription opioids.
Figure 6. Medscape's Test-and-Teach is one example of NIDA's multiplatform approached to enhance a physician's ability to properly manage pain while preventing the abuse of prescription opioids.Because prescription drugs are safe and effective when used properly and are broadly marketed to the public, the notion that they are also harmful and addictive when abused can be a difficult one to convey. Thus, we need focused research to discover targeted communication strategies that effectively address this problem. Reaching this goal may be significantly more complex and nuanced than developing and deploying effective programs for the prevention of abuse of illegal drugs, but good prevention messages based on scientific evidence will be difficult to ignore.[39]
Education is a critical component of any effort to curb the abuse of prescription medications and must target every segment of society, including doctors (Fig.6). NIDA is advancing addiction awareness, prevention, and treatment in primary care practices, including the diagnosis of prescription drug abuse, having established four Centers of Excellence for Physician Information. Intended to serve as national models, these Centers target physicians-in-training, including medical students and resident physicians in primary care specialties (e.g., internal medicine, family practice, and pediatrics). NIDA has also developed, in partnership with the Office of National Drug Control Policy (ONDCP), two online continuing medical education courses on safe prescribing for pain and managing patients who abuse prescription opioids. To date, combined, these courses have been completed over 80,000 times. Additionally, NIDA is directly reaching out to teens with its PEERx initiative, an online education program that aims to discourage prescription drug abuse among teens,[40] by providing factual information about the harmful effects of prescription drug abuse on the brain and body.
NIDA will also continue its close collaborations with ONDCP, the Substance Abuse and Mental Health Services Administration (SAMHSA), and other Federal Agencies. It will also continue to work with professional associations with a strong interest in preserving public health. For example, NIDA recently sponsored a two-day meeting in conjunction with the American Medical Association and NIH Pain Consortium, where more than 500 medical professionals, scientific researchers, and interested members of the public had a chance to dialogue about the problems of prescription opioid abuse and to learn about new areas of research. In another important collaborative effort, NIDA, CDC, SAMHSA, and the Office of the National Coordinator for Health Information Technology reviewed eight clinical practice guidelines on the use of opioids to treat pain and developed a common set of provider actions and associated recommendations.[41]
Conclusion
We are seeing an increase in the number of people who are dying from overdoses, predominantly after abuse of prescribed opioid analgesics. This disturbing trend appears to be associated with a growing number of prescriptions in and diversion from the legal market.
We commend the Subcommittee for recognizing the serious and growing challenge posed by the abuse of prescription and non-prescription opioids in this country, a problem that is exceedingly complex. Indeed, prescription opioids, like other prescribed medications, do present health risks but they are also powerful clinical allies. Therefore, it is imperative that we strive to achieve a balanced approach to ensure that people suffering from chronic pain can get the relief they need while minimizing the potential for negative consequences. We support the development and implementation of multipronged, evidence-based strategies that minimize the intrinsic risks of opioid medications and make effective, long term treatments available.
References
- UNODC, World Drug Report 2012. http://www.unodc.org/unodc/en/data-and-analysis/WDR-2012.html
- Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
- Pradip et al. Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the US. Center for behavioral Health Statistics and QualityData Review. SAMHSA (2013)
IMS’s National Prescription Audit (NPA) & Vector One ®: National (VONA). - International Narcotics Control Board Report 2008. United Nations Pubns. 2009. p. 20
- To clarify our terminology here, when we say “prescription drug abuse” or “nonmedical use,” this includes use of medications without a prescription, use for purposes other than for what they were prescribed, or use simply for the experience or feeling the drug can cause.
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2007: national estimates of drug-related emergency department visits.
- Treatment Episode Data Set (TEDS) Highlights – 2007. National Admissions to Substance Abuse Treatment Services. SAMHSA
- Mack, K.A. Drug-induced deaths - United States, 1999-2010. MMWR Surveill Summ. 2013 Nov 22;62 Suppl 3:161-3. CDC
- Paulozzi et al. Increasing deaths from opioid analgesics in the United States Pharmacoepidemiol. Drug Saf., 15 (2006), pp. 618–627
- Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. REPORT BRIEF JUNE 2011; Johannes et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 11(11):1230-9. (2010); Gallup-Healthways Well-Being Index.
- De Leon Casada. Opioids for Chronic Pain: New Evidence, New Strategies, Safe Prescribing The American Journal of Medicine, 126(3s1):S3–S11. (2013)..
- American Academy of Pain Medicine; American Pain Society; American Society of Addiction Medicine. Definitions Related to the Use of Opioids for the Treatment of Pain. Glenview, IL, and Chevy Chase, MD: American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine; 2001
- Assessment of Analgesic Treatment of Chronic Pain: A Scientific Workshop, linked to 4-24-2014 available at https://www.federalregister.gov/documents/2012/02/08/2012-2757/assessment-of-analgesic-treatment-of-chronic-pain-a-public-workshop-request-for-comment
- ER/LA Opioid Analgesic Class Labeling Changes and Postmarket Requirements (PDF - 136KB)
Letter to ER/LA opioid application holders. Linked to 4-24-2014 Available at http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM367697.pdf (PDF, 139KB) - Mattoo, S. Prevalence and correlates of epileptic seizure in substance-abusing subjects. Psychiatry Clin Neurosci. 63(4):580-2. (2009).
- SAMHSA: Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings and Detailed Tables
- CDC. Vital signs. http://www.cdc.gov/vitalsigns/PrescriptionPainkillerOverdoses/index.html
- Bateman, B.T. et al. Patterns of Opioid Utilization in Pregnancy in a Large Cohort of Commercial Insurance Beneficiaries in the United States. Anesthesiology. in press (2014)
- Coalition Against Insurance Fraud. Prescription for peril: how insurance fraud finances theft and abuse of addictive prescription drugs. Washington, DC: Coalition Against Insurance Fraud; 2007. Available at http://www.insurancefraud.org/downloads/drugDiversion.pdf (PDF, 2MB)
- Centers for Disease Control and Prevention , National Center for Health Statistics. Multiple Cause of Death 1999-2010 on CDC WONDER Online Database, released 2012. Data are from the Multiple Cause of Death Files, 1999-2010, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program.
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2001-2011. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-65, HHS Publication No. (SMA) 13-4772. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
- Brody and Li. Am. J. Epidemiology. 2014
- Williams, J. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 65(1):223-54. (2013).
- Møller et al. Acute drug-related mortality of people recently released from prisons. Public Health. 124(11):637-9. (2010); Buster et al. An increase in overdose mortality during the first 2 weeks after entering or re-entering methadone treatment in Amsterdam. Addiction. 97(8):993-1001. (2002).
- Paulozzi, L. Prescription drug overdoses: a review. J Safety Res. 43(4):283-9 (2012)
- CDC.Vital signs: overdoses of prescription opioid pain relievers and other drugs among women--United States, 1999-2010. MMWR 62(26):537-42. (2013).
- Slevin and Ashburn. Primary care physician opinion survey on FDA opioid risk evaluation and mitigation strategies. J Opioid Manag. 2011 Mar-Apr;7(2):109-15.
Hooten and Bruce. Beliefs and attitudes about prescribing opioids among healthcare providers seeking continuing medical education. J Opioid Manag. 7(6):417-24.(2011). - Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
- SAMHSA advisory Bulletin 2/7/14
Centers for Disease Control and Prevention , National Center for Health Statistics. Multiple Cause of Death 1999-2010 on CDC WONDER Online Database, released 2012. Data are from the Multiple Cause of Death Files, 1999-2010, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. - Moore, A. et al. Expect analgesic failure; pursue analgesic success BMJ. 3;346 (2013).
- Community-Based Opioid Overdose Prevention Programs Providing Naloxone. United States, 2010. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. MMWR. Vol 61/No.6 February 17, 2012.
- NIDA STTR Grantee: AntiOp, Inc., Daniel Wermerling, CEO.
- Schwartz, R.P. et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995-2009. Am J Public Health. 103(5):917-22 (2013).
- Zarkin, G. Benefits and costs of methadone treatment: results from a lifetime simulation model. Health Econ. 14(11):1133-50 (2005).
- Knudsen, H.K.; Abraham, A.J.; and Roman, P.M. Adoption and implementation of medications in addiction treatment programs. J Addict Med 2011; 5:21-27.
- National Institute on Drug Abuse. Principles of Drug Addiction Treatment: A Research-Based Guide (Third Edition), NIH Publication No. 12-4180. Rockville, MD: National Institute on Drug Abuse, 2012. /publications/principles-drug-addiction-treatment
- Spoth et al. Longitudinal substance initiation outcomes for a universal preventive intervention combining family and school programs. Psychology of Addictive Behaviors 16(2):129–134, 2002.
- http://teens.drugabuse.gov/peerx.
- CDC. Home and Recreational Safety. http://www.cdc.gov/HomeandRecreationalSafety/overdose/guidelines.html