Details
Contact
United States
Meeting Summary
The satellite symposium at the World Congress of Psychiatric Genetics, sponsored by the National Institute on Drug Abuse (NIDA) and the National Institute on Alcohol Abuse and Alcoholism, was held on Friday, September 9, 2011, at the Omni Shoreham Hotel in Washington, DC. NIDA Director Nora D. Volkow welcomed participants and attendees and discussed NIDA’s commitment to advancing our understanding of the role of genetics and epigenetics in substance abuse.
Presenters described recent studies on genetics and the draft genome sequence, the role genes play in nicotine and alcohol abuse, and genetic influences on comorbid psychiatric disorders. Keynote speaker Dr. Eric D. Green, Director of the National Human Genome Research Institute, provided a historical overview of epigenetic and genetic research and a look at future directions for genetic and genomic research at the National Institutes of Health (NIH).
Presentations
The Genetic Epidemiology of Substance Abuse
Presenter: Kenneth Kendler, M.D.
Genes have an impact on substance use disorders (SUDs) by increasing the chances that individuals seek out high-risk environments that expose them to substances of abuse and encourage them in their use and misuse. Dr. Kendler described four paradigms of psychiatric genetics, including basic genetic epidemiology, advanced genetic epidemiology, gene finding, and molecular genetics, and reviewed what researchers have learned about each. He also explained the scientific goals of each, including quantifying the differences in a particular disorder in a population that results from genetic differences between individuals, exploring the nature and mode of action related to genetic risk factors, determining the genomic location and the susceptibility of genes, and identifying critical DNA variants and tracing the biological pathways from DNA to disorder.
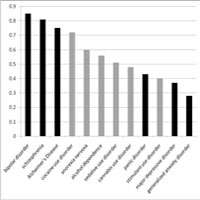
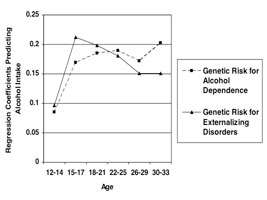
Dr. Kendler explained the role of genes in substance use by discussing research results showing that SUDs are substantially heritable, with estimates ranging between 50 and 60 percent, and that heritability estimates for alcohol disorders (AD) appear to be relatively stable across time and space. Along with highlighting how this estimate compares to other psychiatric disorders, Dr. Kendler reviewed interactions between genes and gender and culture—social factors that have an ability to limit behavior. Research shows that about two-thirds of genetic risk factors for AD and SUDs are not disorder specific, but are shared with other forms of substance abuse and with other externalizing disorders. In early adolescence, siblings’ resemblance for alcohol, nicotine, and cannabis consumption is due to environmental factors; with increasing age, genetic influence seems to play a greater role.
Advanced genetic epidemiology can help answer many of the questions relative to SUDs, including the question of multivariate models and the relationship between genetic and environmental risk factors for SUDs and psychiatric disorders. Genes for AD appear to be moderated by environmental exposures, especially those which relax social constraints, permit easy access to alcohol, or encourage its use. For smoking, his research team found evidence that the impact of genetic risk factors on disease risk depends on the history of environmental exposures. He also explored the gene/environment interaction—that is, the extent to which genetic risk factors and history of environmental exposures interact to contribute to disease risk—by describing a classic example from Swedish adoption studies of alcoholism. This research found that risk is high only in subjects who have a predisposing genetic risk and are also exposed to a high-risk environment. Genetic effects on alcohol use are more pronounced when social constraints are minimized or when the environment permits easy access to alcohol or encourages its use. Last, Dr. Kendler explored the DSM-IV criteria for AD and noted that, from a genetic perspective, they are complex and reflect multiple dimensions of genetic risk.
Genetics of Nicotine Addiction
Presenter: Laura Jean Bierut, M.D.
Dr. Bierut discussed research that has led to a better understanding of the multiple-step progression that leads from never smoked to initiation, becoming a regular smoker, and development of nicotine dependence. Results from the Collaborative Genetic Study of Nicotine Dependence show that of 53,742 screened subjects, roughly half (51 percent) took at least one puff on a cigarette. Of these, more than half (58 percent) went on to smoke at least 100 cigarettes. Of these regular smokers, 44 percent developed nicotine dependence, 35 percent developed some symptoms of dependence, and 19 percent had no dependence symptoms.
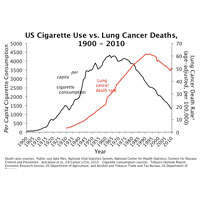
Genetic factors play a role in each stage of this progression. Dr. Bierut discussed results from genome-wide association studies and meta-analyses that have identified multiple loci, sequence variants, and chromosomal regions associated with nicotine dependence and heaviness of smoking. These included genes that influence encoding nicotine-metabolizing enzymes (CYP2A6 and CYP2B6), nicotine acetylcholine receptor subunits (CHRNB3 and CHRNA6), and other genes (CHRNA3, CHRNB4, and CHRNA5) associated with the number of cigarettes smoked per day. The chromosome 15 region including CHRNA5, CHRNA3, and CHRNB4 has the strongest association. She also discussed regions of chromosome 15 that have been identified as having an effect on smoking quantity and other tobacco-related factors and also appear to influence genetic risk for a variety of conditions including schizophrenia, lung cancer, and diabetes.
Genetics of Alcoholism
Presenter: Howard J. Edenberg, Ph.D.
Dr. Edenberg reviewed evidence showing that alcoholism is a complex disease in which risk is affected by genetic variations, the environment, and interactions between them. Most of the variation underlying genetic diseases leads to subtle regulatory differences, not major coding differences, so researchers must look across genes to discover associations. Dr. Edenberg described two exceptional variations in alcohol and aldehyde dehydrogenase enzymes that have a major protective effect against alcohol dependence, and noted that all other genetic variations identified to date have only small effects, which makes identifying variations that affect risk very difficult. He described the strategy of the Collaborative Study on the Genetics of Alcoholism (COGA), a large, family-based study, to identify such genes using many different approaches, from linkage to genome-wide association studies as well as next-generation sequencing and functional studies.
Variations in several genes, including GABRA2 and ADH4, have been identified and replicated. While the research focus has been on common variants expected to have small effects on risk, COGA is now also using next-generation sequencing to study the possible roles of rare variants they have collected. The functions of variants found have been explored by studying phenotypes associated with adolescents and young adults, as well as by studying molecular aspects of gene regulation. The convergence of many approaches will be required to better understand the complex disease of alcoholism.
Functional Genetics
Presenter: Paul Kenny, Ph.D.
Nicotinic acetylcholine receptors are gated ion channels containing five out of 12 possible subunits. Receptors that contain subunits designated alpha 3, alpha 5, and beta 4 are linked to increased susceptibility to nicotine addiction. Dr. Kenny described research that has expanded understanding of how variations in subunit genes influence smoking. He also described current efforts to translate advances in subunit genetics into therapeutic approaches to treat nicotine addiction.
Laboratory studies have shown that mutation in the alpha 5 subunit gene increases nicotine intake in mice. Disrupted signaling via alpha 5 subunits in the medial habenula and interpeduncular nucleus leads to increased nicotine intake in rats and mice. Human studies show an association of a specific set of alpha 5 subunit genes with tobacco addiction severity in schizophrenic patients. Dr. Kenny described current research into development of novel alpha 5 modulators that may help treat tobacco dependence and the identification of new targets for therapeutic drug discovery. He also discussed efforts to assess nicotine intake in knock-in mice carrying human risk alleles.
Epigenetics of Addiction
Presenter: Eric Nestler, Ph.D.
Dr. Nestler described the role of chromatin studies in advancing our knowledge of epigenetic influences on addiction. Chromatin studies have helped identify drug-regulated genes and provided the first-ever look at transcriptional mechanisms in vivo. Studies found distinct temporal properties of drug-induced transcription factors, such as ∆FosB and CREB, in the nucleus accumbens (NAc), a brain region involved in mediating the positive reinforcing effects of substances of abuse. They also found that ∆FosB and CREB mediate distinct aspects of the drug addiction phenotype—namely, drug sensitization versus drug tolerance and dependence, respectively. Researchers first identified mRNAs and noncoding RNAs regulated in NAc by chronic drug exposure. They then overlaid chromatin modifications on regulated mRNAs to improve accuracy of detection and reveal underlying mechanisms. Overlaying transcription-factor binding added even more information on mechanisms. Chronic cocaine use also induces global changes in chromatin modifications in NAc that influence cocaine’s effects on gene expression and behavior. Epigenetic and chromatin research provide an unprecedented view of the transcriptional mechanisms underlying chronic drug action in NAc and may lead to novel diagnostic and treatment approaches for drug addiction.
The Future is Bright: Charting a Course for Genomic Medicine
Presenter: Eric D. Green, M.D., Ph.D.
In his keynote address, Dr. Green provided a historical overview of human genome sequence research, its role in the future application to healthcare and medicine, and the current state of genomic studies. The Human Genome Project (HGP) began in October 1990 and produced the first draft sequence at a White House event in June 2000. In 2001, the HGP consortium published the first analysis of the human genome in Nature. HGP delivered the final human reference sequence in April 2003, the 50th anniversary of the publication of the double helix. Since 2003, NIH and the National Human Genome Research Institute (NHGRI) have used the reference sequence as a foundation on which to build new research programs. For example, NIH and NHGRI researchers, along with international research groups, conduct genome-wide association studies and epigenome-wide association studies to identify genomic pathways that contribute to complex diseases. NIH investment in genomic and epigenomic research is widely distributed—more than 90 percent of NIH funding indexed by keywords “genome” or “genomics” was spent outside NHGRI. In February 2011, NHGRI published a new vision for genomics research that will lead “from base pairs to bedside”—to healthcare tailored to the individual based on genomic information.