In contrast to cocaine treatment medication development strategies that target dopamine receptors, the brain cell molecules that are overstimulated during cocaine use, other NIDA-funded researchers are investigating ways to neutralize the drug in the bloodstream, reducing the amount available for brain uptake. A main benefit of this approach would be that adverse side effects associated with medications aimed at dopamine receptors, such as disruptions in motor functions, could be avoided. By attacking cocaine directly, this approach also could help reverse some of the drug's toxic effects, such as decreased blood flow and reduced oxygen delivery to the brain.
The primary cocaine-neutralizing tools under investigation are catalytic antibodies, synthetic molecules developed within the past decade by a process involving both synthetic chemistry and immunology. Like natural antibodies, catalytic antibodies are designed to recognize, and bind to, a specific molecule. But unlike naturally occurring antibodies, catalytic antibodies function as enzymes by inducing the molecules to which they bind to undergo a chemical reaction.
Although this research is still in an early stage of development, some researchers envision catalytic antibodies that bind to cocaine in the bloodstream and enzymatically break it down into its nonaddictive components-mimicking the body's natural metabolism of cocaine, but at a much faster rate. The first step toward this goal was reported in 1993 by Dr. Donald Landry, a researcher at the Columbia University College of Physicians and Surgeons in New York, who created a catalytic anti that was able to break cocaine down in test-tube experiments.
Theoretically, catalytic antibodies are potentially powerful new weapons against cocaine abuse. But even enthusiastic proponents of this approach say that it will take at least several more years of research to determine whether catalytic antibodies can be a viable treatment alternative for cocaine abuse.
"In the next year or two, we're probably not going to see a humanized catalytic antibody that can detoxify cocaine or any other drug of abuse," says Dr. John R. Cashman, a NIDA-funded researcher currently working on these compounds. A humanized antibody, he explains, is one that is able to work safely and effectively in the human body. "But what is important," he adds, "is to continue to try to develop new detoxification catalysts that do not currently exist in nature."
Dr. Cashman, a senior scientist at the Seattle Biomedical Research Institute, says that catalytic antibodies ultimately could be used to rescue individuals from acute cocaine overdose. If they work as planned, these specially designed catalysts would quickly reduce the amount of cocaine circulating in the bloodstream of people who overdose.
The plausibility of treating drug addiction with antibodies, an approach known as immunotherapy, was demonstrated in 1974 by former NIDA Director Dr. Charles R. Schuster, currently a professor and director of the Substance Abuse Clinical Research Program at Wayne State University in Detroit, Michigan. Dr. Schuster showed that antibodies to heroin could block its effects in addicted monkeys. This approach, however, only worked for very low doses of heroin since a heroin antibody can only bind to, and neutralize, a single heroin molecule. Administering the large doses of antibody that would be needed to neutralize large doses of heroin is impractical in a clinical setting.
Catalytic antibodies improve on the earlier approach to immunotherapy because the antibodies are not bound permanently to their target molecule. Instead, they are "turned over." After they bind and catalyze the enzymatic breakdown of their target, catalytic antibodies are released and can then bind to another target molecule (see figure below). Turnover thus prevents the rapid depletion of the antibodies.
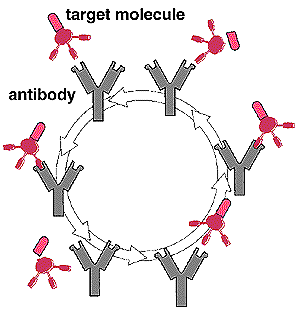 Scientists are trying to develop catalytic antibodies, synthetic molecules that will target and break down cocaine molecules more quickly than the body's natural cocaine-metabolizing enzymes. This simplified diagram shows how a typical catalytic antibody works. The antibody recognizes (upper left), binds to, and induces the breakdown of a specific target molecule. With the release of the breakdown byproducts, the antibody is recycled and is free, unlike naturally occurring antibodies, to repeat this process with another target molecule.
Scientists are trying to develop catalytic antibodies, synthetic molecules that will target and break down cocaine molecules more quickly than the body's natural cocaine-metabolizing enzymes. This simplified diagram shows how a typical catalytic antibody works. The antibody recognizes (upper left), binds to, and induces the breakdown of a specific target molecule. With the release of the breakdown byproducts, the antibody is recycled and is free, unlike naturally occurring antibodies, to repeat this process with another target molecule.Still, some researchers suggest that it is too early to attempt to develop catalytic antibody medications for cocaine abuse. They point to the formidable technical hurdles that must be overcome before these compounds can be clinically useful as evidence that more basic research is needed.
"It is proving extremely challenging to develop a catalytic antibody with a turnover rate that even approaches the turnover rates of natural cocaine metabolizing enzymes," says Dr. Michael Owens, a NIDA grantee who also is investigating immunotherapeutic approaches for drug abuse. "Therefore, we should first determine if similar approaches, which are more technically feasible, might serve the same purpose."
Dr. Owens, a professor of pharmacology and toxicology at the University of Arkansas College of Medicine, says that other approaches might include purifying natural cocaine-metabolizing enzymes from human blood or developing monoclonal, or genetically identical, antibodies that bind to cocaine molecules with a high degree of specificity.
"Nevertheless," Dr. Owens adds, "basic research on cocaine catalytic antibodies should be pursued since the development of pharmacokinetic and metabolic modifiers of abused drugs is an under explored area of medications development."
Dr. Cashman concurs on the need for additional basic research but adds that this should not preclude current efforts to develop clinically useful catalytic antibodies.
"NIDA should be commended for embracing this kind of research activity," says Dr. Cashman.
