In addition to the neuroimaging research being conducted at Division of Intramural Research, NIDA-supported research across the Nation also is offering a greater understanding of addiction through the use of sophisticated neuroimaging techniques. The studies range from identifying brain structures involved in craving to mapping the emotional circuitry of substance abusers to imaging the neurobiological basis of drug-induced euphoria.
"Neuroimaging allows us to see what is going on in a drug addict's brain when he or she craves a drug or after he or she has taken it," says Dr. Joseph Frascella, chief of NIDA's Etiology and Clinical Neurobiology Branch. "We are still in the early stages of this research, and the techniques are being refined almost daily, but the findings are striking and the studies get at the core of NIDA's mission - to understand and modify the effects of drugs of abuse." (For more about how these imaging techniques work, see The Basics of Brain Imaging)
PET Studies
Several NIDA-funded researchers are using positron emission tomography (PET) to study aspects of drug abuse such as craving and the biochemical effects of drugs on the brain. For example, Dr. Anna Rose Childress at the Addiction Research Center at the University of Pennsylvania School of Medicine in Philadelphia and her colleagues have used PET to image the brain's limbic system when craving occurs in cocaine abusers. The limbic system is involved in the rewarding effects of abused drugs and is believed to be important in learning to connect rewards to the cues that signal them.
Dr. Childress presented her findings most recently at the 1996 Annual Meeting of the Society for Neuroscience. The researchers prompted craving in study participants with a videotape showing scenes of cocaine paraphernalia. Measuring blood flow in different regions of the brain through PET, they found that particular areas of the limbic system "light up," or are activated, among cocaine abusers experiencing craving, an indication of increased brain activity.
"Our findings suggest that cocaine craving activates this circuitry to an exceptionally high level," which results in craving, a very strong learned response with powerful motivational properties, Dr. Childress says. Cues that signal cocaine pull the drug abuser toward drug use, she says. Similar PET studies of craving are being conducted at NIDA's Division of Intramural Research. (See NIDA Brain Imaging Research Links Cue-Induced Craving to Structures Involved in Memory)
One goal of the imaging studies is to develop medications that block craving.The researchers could use PET to help determine a medication's effectiveness and how often it should be given, as well as allow researchers to search for possible side effects.
In 1988, Dr. Nora D. Volkow, chairman of the Medical Department and director of nuclear medicine at Brookhaven National Laboratory in Upton, New York, became one of the first researchers to demonstrate, using PET, that cocaine produces vascular disease in the brain. Today, she continues a wide range of drug abuse research using PET. On the basis of her recent findings, Dr.Volkow proposes that cocaine's powerful effects may be due in part to the rapid rate at which the drug acts on and then leaves the brain. Other drugs that activate the same areas are removed more slowly and do not have the same powerful abuse potential. The drug's fast action accounts for its highly reinforcing properties, and its fast removal promotes frequent use, she says.
Dr. Volkow and her colleagues believe that this frequent administration of the drug activates a brain circuit that is involved in a range of behaviors such as eating that, biologically, require repetition. Usually, this circuit is deactivated once the behavior has achieved its goal. However, Dr. Volkow's recent findings suggest that, in the case of cocaine, chronic use of the drug keeps the circuit "turned on," producing an almost perpetual compulsion to use the drug.
Like Dr. Childress's research, Dr. Volkow's studies have the ultimate goal of speeding development of cocaine treatment medications. "Better understanding of a drug's effects suggests new treatment strategies," says Dr. Volkow.
Dr. George Ricaurte, an assistant professor in the Department of Neurology at the Johns Hopkins Medical Institutions in Baltimore, and his colleagues are using PET to study possible long-term effects of amphetamine derivatives in the brain. Drugs under investigation include methamphetamine; methcathinone, a methamphetamine-like drug commonly known as "cat" that has recently appeared on the street; and MDMA (3,4-methylenedioxymethamphetamine), known among drug users as "ecstasy."
The preliminary evidence regarding methcathinone indicates that people who take repeated doses show lasting reductions in brain cell activity that produces the chemical messenger dopamine. If these effects are permanent,and there is an age-related decline in brain dopamine, methcathinone abusers may be at increased risk of developing problems such as parkinsonism, which is caused by a dopamine deficiency, Dr. Ricaurte says.
"The public health question here is whether people who use methamphetamine and related drugs are incurring brain injury similar to that observed in animals and whether there are clinical consequences of such injury," says Dr. Ricaurte.(For more about Dr. Ricaurte's studies, see Response to Escalating Methamphetamine Abuse Builds on NIDA-Funded Research, and Like Methamphetamine, 'Ecstasy' May Cause Long-Term Brain Damage.)
MR Studies
Other NIDA-funded researchers are using magnetic resonance (MR) imaging and spectroscopy to observe how brain structures change as drugs are producing their effects. MR is especially well suited for measuring changes, says Dr. Perry Renshaw, research director of the Brain Imaging Center at McLean Hospital in Belmont, Massachusetts, and an assistant professor at Harvard Medical School. "We can scan patients over and over, including during treatment, and the technique is relatively inexpensive," he says.
Dr. Renshaw and his colleagues are using phosphorus magnetic resonance spectroscopy(31P MRS), another type of functional imaging, to measure abnormal brain activity in drug abusers.
When brain cells are active, their increased metabolism generates compounds that contain phosphorus. 31P MRS can determine which areas of the brain are active or damaged by measuring phosphorus levels in brain tissue. Dr.Renshaw and his colleagues are seeing that chronic drug abuse is accompanied by abnormal brain metabolism in some areas of the brain. The brain metabolism of drug abusers seems to improve when they stop using drugs, he says.
Dr. Hans Breiter, a psychiatrist in the Departments of Psychiatry and Radiology at Massachusetts General Hospital, and his colleagues are using functional magnetic resonance imaging (fMRI) to study the direct effects of cocaine on brain function. They are observing what parts of the brain are active when an addict is feeling high from cocaine and what parts of the brain are active when an addict craves more cocaine after having used the drug."We need to understand the craving felt by the cocaine user when the drug effects are wearing off and the user wants more cocaine, if we are to develop effective treatment for cocaine use," says Dr. Breiter. He and his colleagues have found that the nucleus accumbens, a structure deep inside the brain that is important in experiencing rewards, "lights up" during episodes of this type of craving.
Dr. Breiter and colleagues also are investigating the amygdala, another brain structure, which other researchers have shown is activated during cue-induced cocaine craving. "It is believed that the amygdala plays an important role in determining what is emotionally significant in the environment," he says.
Usually, people habituate or mentally adjust to objects in the environment that produce an emotional response, Dr. Breiter explains. He is studying the role of the amygdala in this process and memory-related processes when normal subjects are shown faces with happy expressions or fearful expressions. "We found that activation of the amygdala in response to emotional faces rapidly decreased over time, suggesting less attention to these emotional cues,"says Dr. Breiter. "These imaging studies are helping us understand the basis of emotion and motivation, which may help us understand how drugs affect the brain."
 Dr. Linda Chang of Harbor-UCLA Medical Center combines MRI, MRS, SPECT, and perfusion MRI images using a computer program written at the Center.
Dr. Linda Chang of Harbor-UCLA Medical Center combines MRI, MRS, SPECT, and perfusion MRI images using a computer program written at the Center.Combining Technologies
Some NIDA-funded researchers are combining techniques to take advantage of the strengths of each. For example, neurologist Linda Chang and her colleagues in the Department of Neurology at the Harbor-UCLA Medical Center in Torrance, California, are using MRI, MRS, single photon emission computed tomography(SPECT), and perfusion MRI to study the effect of cocaine on AIDS dementia complex, a loss of mental and physical ability caused by infection of the brain by HIV, the virus that causes AIDS. She is also using MRI, MRS, and SPECT to study the neurotoxicity of the drug "ecstasy." Further, Dr. Chang and her colleagues are applying these technologies to study ethnic and gender differences in cocaine's effects. It appears that cocaine affects the brain chemistry of men and women differently, she says.
Dr. Scott E. Lukas, director of the clinical neuropsychopharmacology laboratory at McLean Hospital and an associate professor of psychiatry at Harvard Medical School, is using electroencephalograms (EEGs) and MRI together to produce the equivalent of a topographic map of the brain that shows an increase in alpha waves produced by cocaine. Alpha waves are a particular frequency of electrical activity in the brain that indicate a relaxed state or inactivity. Mapping alpha waves during cocaine use shows what the brain looks like when the drug abuser feels cocaine-induced euphoria, Dr. Lukas says.
These EEG measurements are used to produce the coordinates that pinpoint where these alpha waves are coming from in the brain. Next, Dr. Lukas images the patient's brain with MRI, which shows brain structures, and matches this image with the coordinates. The researchers are finding that a source of cocaine-induced euphoria is the thalamus, a major relay center for sensory information that both receives and sends out messages.
This investigation of alpha wave activity suggests a potential clinical value for medications that could produce alpha waves at lower levels for longer periods of time. "Presumably, these medications would be less likely to be abused because they produce less euphoria," says Dr. Lukas.
"Neuroimaging has rapidly become one of the key features of research in neuroscience," says NIDA's Dr. Frascella. These studies allow scientists to really understand the brain processes that are altered by drugs, he believes. NIDA expects many important findings and treatment applications to emerge from these and other NIDA-funded imaging studies, he says.
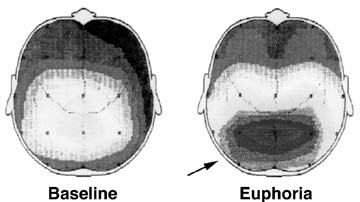 Dr. Scott Lukas and his colleagues at McLean Hospital and Harvard Medical School combined electroencephalograms and MRI to create this topographic brain map. The image at the right shows the increase in alpha wave activity during cocaine-induced euphoria.
Dr. Scott Lukas and his colleagues at McLean Hospital and Harvard Medical School combined electroencephalograms and MRI to create this topographic brain map. The image at the right shows the increase in alpha wave activity during cocaine-induced euphoria.Sources:
- Breiter, H., et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17:875-887, 1996.
- Breiter, H., et al. Activation of human brain reward circuitry by cocaine observed using fMRI. Proceedings of the Society for Neuroscience 3:1933,1996.
- Chang, L. In vivo magnetic resonance spectroscopy in HIV and HIV-related brain diseases. Reviews in the Neurosciences 6(4):365-378, 1995.
- Chang, L.; Ernst, T.; and Strickland, T. Neurochemical abnormalities and gender effects in abstinent asymptomatic cocaine users. Proceedings of the InternationalSociety for Magnetic Resonance in Medicine 2:992, 1996.
- Childress, A.R.; Mozley, P.D.; Fitzgerald, J.; Reivich, M.; Jaggi, J.; andO'Brien, C.P. Regional brain blood flow during cue-induced cocaine craving. Society for Neuroscience Abstracts 20:666.2, 1994.
- Childress, A.R.; Mozley, P.D.; Fitzgerald, J.; Reivich, M.; Jaggi, J.; andO'Brien, C.P. Limbic activation during cue-induced cocaine craving. Society for Neuroscience Abstracts 21:767.1, 1995.
- Childress, A.R.; McElgin, W.; Mozley, D.; Reivich, M.; and O'Brien, C.P.Brain correlates of cue-induced cocaine and opiate craving. Society forNeuroscience Abstracts 22:365.5, 1996.
- Christensen, J.D.; Kaufman, M.J.; Levin, J.M.; Mendelson, J.H.; Holman,B.L.; Cohen, B.M.; and Renshaw, P.F. Detection of abnormal cerebral metabolism in polydrug abusers during early withdrawal using 31P MR spectroscopy. Magnetic Resonance in Medicine 35:658-663, 1996.
- Grob C.; Poland, R.; Chang, L.; and Ernst, T. Psychobiologic effects of3,4-methylenedioxymethamphetamine (MDMA) in humans; methodological considerations and preliminary observations. Behavioral Brain Research 73:103-107, 1996.
- Lukas, S.E. Advanced electrophysiological imaging techniques for studying drug effects. In: London, E.D., ed. Imaging Drug Action in the Brain. Boca Raton, FL: CRC Press, Inc., pp. 389-404, 1993.
- Ricaurte, G.; Wong, D.; Szabo, Z.; Yokoi, F.; Scheffel, U.; Mathews, W.;Ravert, H.; and Dannals, R. Reductions in brain dopamine and serotonin transportersdetected in humans previously exposed to repeated high doses of methcathinoneusing PET. Society for Neuroscience Abstracts 22:1915, 1996.
- Sparago, M.; Wlos, J.; Yuan, J.; Hatzidimitriou, G.; Tolliver, J.; Dal Cason,T.A.; Katz, J.; and Ricaurte, G. Neurotoxic and pharmacologic studies onenantiomers of the N-methylated analog of cathinone (methcathinone): A newdrug of abuse. Journal of Pharmacology and Experimental Therapeutics 279(2):1043-1052,1996.
- Volkow, N.D.; Ding, Y.-S.; Fowler, J.S.; and Wang, G.J. Cocaine addiction:Hypothesis derived from imaging studies with PET. Journal of Addictive Diseases15:55-71, 1996.
