Recent studies with genetically altered mice have suggested that cocaine's euphoric effects may involve not just one, but several, chemical sites in the brain. These studies indicate that medications for treating cocaine addiction may need to target these multiple sites just as cocaine does.
Scientists have known for many years that cocaine blocks the reuptake of certain chemicals by nerve cells, or neurons, in the brain. Neurons release these chemicals, called neurotransmitters, to send messages to other neurons in the vicinity. Once communication has taken place, the neurons that sent the neurotransmitters recycle them for further use. Proteins called transporters, located on the surface of the sending neurons, latch onto the neurotransmitters outside the neurons in the extracellular space and transport them back inside for re-release at a later time.
Early studies showed that cocaine blocks the transporters for three different neurotransmitters: dopamine, serotonin, and norepinephrine. Later, one vein of research suggested that cocaine's blockade of the dopamine transporter was most important for producing the drug's euphoric effects. By blocking the dopamine transporter, some scientists theorized, cocaine might raise the level of extracellular dopamine in brain regions involved in the feeling of pleasure. This excess dopamine could continue to affect neurons in these regions, giving rise to euphoria.
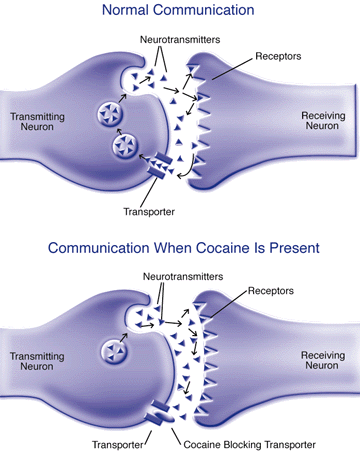 Cocaine Disrupts Communication Between Neurons. During normal communication between nerve cells, or neurons, the transmitting neuron releases neurotransmitter molecules that stimulate the receiving neuron by binding to receptor molecules on its surface. After this communication has occurred, transporter molecules collect the released neurotransmitters and transport them back into the transmitting neuron for later release. When cocaine is present, the drug blocks the transporter, preventing neurotransmitter reuptake so that the neurotransmitters continue to stimulate the receiving neuron.
Cocaine Disrupts Communication Between Neurons. During normal communication between nerve cells, or neurons, the transmitting neuron releases neurotransmitter molecules that stimulate the receiving neuron by binding to receptor molecules on its surface. After this communication has occurred, transporter molecules collect the released neurotransmitters and transport them back into the transmitting neuron for later release. When cocaine is present, the drug blocks the transporter, preventing neurotransmitter reuptake so that the neurotransmitters continue to stimulate the receiving neuron.If this hypothesis is true, then eliminating the dopamine transporter in the brain should eliminate cocaine's euphoric effects. To test the hypothesis, scientists produced mice lacking dopamine transporters by inactivating or "knocking out" the gene for the transporter in mouse embryos. When these dopamine transporter "knockout" mice matured, the researchers studied whether they found cocaine to be rewarding. Researchers used two techniques to study whether elimination of the dopamine transporter nullified cocaine's rewarding effects.
Dr. Beatriz Rocha, then at the University of North Texas Health Science Center in Fort Worth and now in NIDA's Intramural Research Program (IRP) in Baltimore, and Dr. Marc Caron's group at Duke University in Durham, North Carolina, used a procedure in which the mice pressed a lever to receive a cocaine injection. If the mice continually pressed the lever at a high rate, this would indicate that they found cocaine rewarding.
Dr. Ichiro Sora, Dr. George Uhl, and their colleagues in NIDA's IRP, the IRP of the National Institute of Mental Health in Bethesda, Maryland, and the University of WŸrzburg in Germany used a different procedure called conditioned place preference. In this procedure, mice were given cocaine injections when they were in one compartment of a two-compartment chamber and were given nothing when they were in the other compartment. Later, the researchers would observe which compartment the mice moved to when they were given a choice. If the mice found cocaine rewarding, they would spend more time in the compartment where they had received the cocaine injections.
Using the different procedures, both groups found that their knockout mice found cocaine rewarding despite not having the dopamine transporter. The mice either self-administered cocaine or chose the side of the cage where they had received cocaine.
"This finding surprised us at first," says Dr. Uhl. "It shows that the dopamine transporter is not necessary for cocaine reward." Dr. Rocha says that she, too, was surprised by her findings, but the fact that she and her colleagues and Dr. Uhl's group had complementary results adds weight to the findings.
If the dopamine transporter is not the crucial site for producing cocaine reward, then what is? Apparently not the serotonin transporter, because Dr. Uhl's group also studied serotonin transporter knockout mice and found that these mice also found cocaine rewarding.
Dr. Uhl and Dr. Rocha speculate that perhaps cocaine produces its rewarding effects by blocking the dopamine transporter and the serotonin transporter at the same time. Thus, the elevation in the levels of both dopamine and serotonin might produce the feelings of pleasure.
In Dr. Rocha's study, the researchers found that the extracellular dopamine level in a key brain region in the dopamine transporter knockout mice was nearly five times higher than normal because the transporters were no longer there to shuttle the dopamine molecules back inside the neurons. When the knockout mice were given cocaine, the extracellular dopamine level did not go any higher because the animals had no dopamine transporters for cocaine to block. Although the researchers have yet to measure the extracellular serotonin levels in these knockouts, Dr. Rocha figures that the levels increased and then decreased as other studies have shown they do in normal mice because knocking out the dopamine transporter probably would not affect cocaine's blockade of the serotonin transporter. (See "Effects of Cocaine on Neurotransmitter Levels." below)
The increase in serotonin, combined with the already high level of dopamine, may be why cocaine is rewarding for the dopamine transporter knockout mice, according to Dr. Rocha.
Dr. Uhl also believes that more than one neurotransmitter in the brain probably mediates cocaine reward, if only because more than one neurotransmitter probably mediates pleasure in general. "If a species is not rewarded by activities such as eating or sexual interactions, that species is not going to survive," he says. "So it makes sense that the brain would have redundant systems so that if a mutation or some other factor disrupts one system, the other systems can still operate normally to produce reward." Different neurotransmitters might mediate different aspects of reward, he says. The explanation for cocaine's powerful attraction may be that it affects several neurotransmitters, all of which are involved in mediating pleasure.
Selective or Nonselective?
Both Dr. Uhl and Dr. Rocha think that the results of their dopamine transporter knockout studies support the idea that medications for treating cocaine addiction should target other neurotransmitters in addition to dopamine. Dr. David McCann, chief of NIDA's Pharmacology and Toxicology Branch, notes that starting in the early 1990s NIDA, in collaboration with pharmaceutical firms, began developing a number of potential cocaine treatment medications that prevent cocaine from acting at neurotransmitter transporters. Some of these compounds are selective for the dopamine transporter, while others act more or less equally at dopamine, serotonin, and norepinephrine transporters.
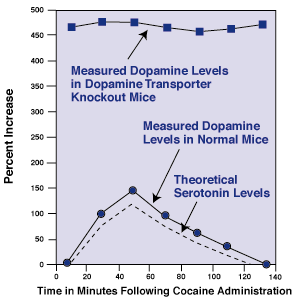 Effects of Cocaine on Neurotransmitter Levels. In normal mice, researchers have found that cocaine raises the levels of dopamine and serotonin outside neurons about 150 percent, but the levels return to normal after about 2 hours. In Dr. Rocha's study, the researchers found that the dopamine levels in dopamine transporter knockout mice were about 500 percent higher than normal because no transporters were available to shuttle the dopamine molecules back inside the neurons.
Effects of Cocaine on Neurotransmitter Levels. In normal mice, researchers have found that cocaine raises the levels of dopamine and serotonin outside neurons about 150 percent, but the levels return to normal after about 2 hours. In Dr. Rocha's study, the researchers found that the dopamine levels in dopamine transporter knockout mice were about 500 percent higher than normal because no transporters were available to shuttle the dopamine molecules back inside the neurons.Although the researchers have yet to measure the extracellular serotonin levels in these knockouts, Dr. Rocha theorizes that the levels increase then decrease as in normal mice because knocking out the dopamine transporter probably would not affect cocaine's blockade of the serotonin transporter.
A compound that is selective for the dopamine transporter is GBR12909 (see "Compounds Show Strong Promise For Treating Cocaine Addiction," NIDA NOTES, May/June 1997.). A compound that blocks all three transporters about equally is NS2359, which was developed by NIDA and NeuroSearch, a Danish pharmaceutical firm. Animal studies with these compounds have indicated that they are safe and potentially effective in humans, and they are now in the early phases of human clinical trials.
Sources
- Rocha, B.A.; Fumagalli, F.; Gainetdinov, R.R.; Jones, S.R.; Ator, R.; Giros, B.; Miller, G.W.; and Caron, M.G. Cocaine self-administration in dopamine-transporter knockout mice. Nature Neuroscience 1(2):132-137, 1998.
- Sora, I.; Wichems, C.; Takahashi, N.; Li, X-F; Zeng, Z.; Revay, R.; Lesch, K-P; Murphy, D.L.; and Uhl, G.R. Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proceedings of the National Academy of Sciences USA 95(13):7699-7704, 1998.
