At the culmination of a research journey that began more than 20 years ago, GVG (vigabatrin)--a medication widely used to treat epilepsy outside the United States--is about to be tested in large-scale clinical trials that will determine whether its promising pharmacological properties can translate into effective treatment medication for cocaine abuse. Winning FDA approval of any pharmaceutical therapy for use in the United States is an exacting, costly, time-consuming process. It involves lengthy research, testing in animals and, finally, testing in increasingly larger groups of selected, medically suitable humans to show that the drug is not only therapeutically effective, but safe to use. Testing medications to treat drug addiction is especially challenging, as it involves recruiting--and gaining the support and cooperation of--drug-addicted people.
"Developing a medication to treat cocaine addiction has long been a research priority of NIDA's Medications Development Program. According to the National Survey on Drug Use and Health, an estimated 2 million people were current cocaine users in 2002. The available treatment offerings for these individuals as well as people addicted to methamphetamine and other stimulant drugs are exclusively behavioral, as no treatment medications have yet proven effective."
- Dr. Frank Vocci, Director, Division of Treatment Research and Development
After more than 20 years, it appears that GVG is nearing the final hurdles to winning FDA approval for use in treating cocaine addiction. Detailed below is this long journey, not untypical of the process many medications go through before providing relief to their intended recipients.
Identifying GVG's Promise
In the 1980s, Dr. Stephen L. Dewey of Brookhaven National Laboratory in Upton, New York, and a colleague, Dr. Jonathan D. Brodie of the New York University School of Medicine in New York City, were seeking new treatments for schizophrenia. "Very few people in the mid-1980s looked at interactions between neurotransmitter systems, but rather examined transmitter systems independently," explains Dr. Dewey. As studies continued over years and preliminary research showed that GVG modulates GABA, which in turn reduces dopamine levels, the scientists launched a long series of preclinical experiments testing GVG's potential as a treatment medication for addiction.
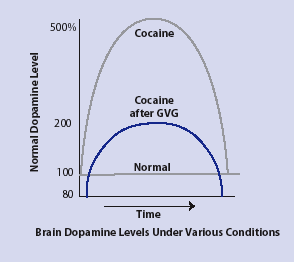 GVG Blocks Cocaine-Triggered Dopamine Increases in Animals Cocaine increases dopamine levels in animals to as much as 500 percent of normal levels in critical brain areas. However, when animals are given GVG before being given cocaine, their dopamine levels increase to no more than twice normal levels, indicating the reward-reinforcement response has been blocked or greatly reduced.
GVG Blocks Cocaine-Triggered Dopamine Increases in Animals Cocaine increases dopamine levels in animals to as much as 500 percent of normal levels in critical brain areas. However, when animals are given GVG before being given cocaine, their dopamine levels increase to no more than twice normal levels, indicating the reward-reinforcement response has been blocked or greatly reduced."Two decades and 15 publications later, we knew that GVG can safely block the biochemical effects of addictive drugs--nicotine, morphine, methamphetamine, amphetamine, ecstasy, and alcohol--including the increased brain dopamine levels they trigger," Dr. Dewey says. "This medication can also block the behavioral fallout from dopamine surges: drug self-administration, changes in brain-stimulation reward threshold, relapse resulting from addiction-induced cues, and drug sensitization--the need to consume more and more of a drug to achieve dopamine-based feelings of pleasure."
With evidence of GVG's ability to block the addictive effects of all psychostimulants, the researchers focused their research on its potential as a pharmaceutical therapy for cocaine addiction. Throughout this research, Dr. Dewey charted how dramatically GVG therapy inhibits cocaine-induced increases in brain dopamine levels in animals. However, plans to test GVG as a treatment for cocaine abuse in humans were sidetracked in 1998, when research showed that 10 to 30 percent of epilepsy patients taking the drug lost some portion of their normal field of vision.
Meeting a Safety Challenge
Not willing to give up on GVG's potential, Dr. Dewey and his Brookhaven colleagues set out to find a low-dosage course of GVG that could effectively block the addictive responses of cocaine in rats without impairing their vision. The researchers' strategy: compare the effects of a single large dose of 150-450 mg/kg GVG with those of the same total dose administered over 3 days, at 50-150 mg/kg per day. The scientists found that the anti-addictive effect of GVG persists when it is administered gradually. The drug inhibited the effect of cocaine after a 3-day "washout" period when GVG administration stopped. Further, the inhibitory effect of gradual administration actually exceeded in magnitude and duration the effect of the identical total one-shot dose, the researchers found.
The researchers concluded that people can probably sustain GVG maintenance therapy, possibly for years, with little or no increased risk of developing medication-induced vision defects.
Launching Clinical Trials
Using human dosages based on the GVG dosages that safely countered cocaine's effect in rats and primates, Drs. Dewey and Brodie launched a clinical trial with 20 cocaine abusers in a drug treatment center directed by Dr. Emilia Figueroa in Mexicali, Mexico. Following the dosing strategy devised for animals, the researchers introduced GVG therapy with a strategy of "ramping the patients' doses up, and then tapering them off," explains Dr. Dewey.
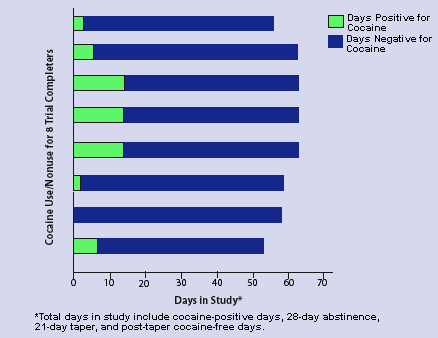 Cocaine Users Achieve Abstinence, Remain Drug-Free With GVG Of 20 cocaine abusers enrolled in a GVG clinical trial, 8 completed the trial (28-day abstinence plus 21-day taper) and were drug-free for 46 to 58 days total. Once cocaine use ceased, 6 of the 8 completers were entirely drug-free for the duration of the study. Most patients who completed the trial reported that their craving for cocaine stopped 2 to 3 weeks after receiving GVG.
Cocaine Users Achieve Abstinence, Remain Drug-Free With GVG Of 20 cocaine abusers enrolled in a GVG clinical trial, 8 completed the trial (28-day abstinence plus 21-day taper) and were drug-free for 46 to 58 days total. Once cocaine use ceased, 6 of the 8 completers were entirely drug-free for the duration of the study. Most patients who completed the trial reported that their craving for cocaine stopped 2 to 3 weeks after receiving GVG.Of the 20 patients enrolled in the study, 8 remained in the program and were drug-free for periods of 46 to 58 days. Twelve enrollees failed to complete the clinical trial; of these, 8 dropped out within the first 10 days, having decided they were not ready to stop abusing cocaine. Four enrollees participated in the study for 25 to 43 days while continuing to abuse cocaine, albeit in greatly reduced amounts. Most patients who completed the trial reported losing their craving for cocaine after 2 to 3 weeks of GVG. Once they stopped cocaine abuse, 6 of the 8 completers were drug-free for the duration of the study. No vision problems were reported.
Encouraged by the Mexico results, the researchers look forward to the next step--a larger, double-blind, placebo-controlled trial that they hope to initiate soon in Toronto, Canada. Catalyst Pharmaceutical Partners, which holds the license from Brookhaven to develop GVG as a treatment for drug addiction, has helped move this trial forward. Another smaller trial is also being projected for the United States. Dr. Frank Vocci, Director of NIDA's Division of Treatment Research and Development, says that NIDA is ready to cooperate once FDA gives its approval to go ahead.
"We are still early in the clinical trial process," notes Dr. Vocci. "However, if additional trials safely and successfully replicate early treatment results, GVG has the potential to provide us with an effective medication for preventing relapse in cocaine-dependent patients."
Commenting on the impressive GVG research record, Dr. Vocci concludes, "The data these researchers have compiled is the most comprehensive on a potential medication for addiction therapy that we've seen," he says. "It's a very interesting medication that involves a very interesting mechanism."
Sources
- Brodie, J.D.; Figueroa, E.; and Dewey, S.L. Treating cocaine addiction: From pre-clinical to clinical trial experience with γ-vinyl GABA. Synapse 50(3):261-265, 2003. [Abstract]
- Schiffer, W.K.; Marsteller, D.; and Dewey, S.L.. Sub-chronic low dose γ-vinyl GABA (vigabatrin) inhibits cocaine-induced increase in nucleus accumbens dopamine. Psychopharmacology 168(3):339-343, 2003. [Abstract]
