In the past few decades, scientists have firmly established that the desire to take drugs has a biological basis in fluctuations in levels of the brain chemical dopamine. Now, a NIDA-supported experiment with laboratory animals strongly suggests that dopamine has a second, different but comparably critical role in prompting individuals to translate that desire into action.
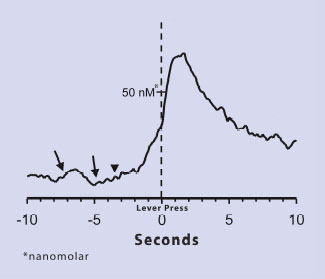 Anticipation of Cocaine Triggers Dopamine Increase in Rats. Rats trained to self-administer cocaine exhibited elevations in dopamine concentrations when they anticipated cocaine and again when they began to seek the drug. Arrows indicate when dopamine levels began to increase; the inverted triangle shows when rats approached the lever that triggered a cocaine infusion.
Anticipation of Cocaine Triggers Dopamine Increase in Rats. Rats trained to self-administer cocaine exhibited elevations in dopamine concentrations when they anticipated cocaine and again when they began to seek the drug. Arrows indicate when dopamine levels began to increase; the inverted triangle shows when rats approached the lever that triggered a cocaine infusion.People hanker for drugs largely to re-experience the intense pleasure they recall from past episodes of drug abuse. Much research has shown that this pleasure occurs because drugs of abuse--each by its own pharmacological mechanism--precipitate surges of dopamine in a part of the brain called the nucleus accumbens (NAc). In drug abuse as in other parts of life, however, there is a difference between desiring something and actually taking steps to achieve it. Chronic drug abusers often avoid acting on their drug-seeking urge for long periods. If their resolve to abstain fails, it often does so when they encounter something or someone they associate with past drug taking. The new study suggests that like drugs themselves, such encounters produce surges in dopamine levels, and these surges push the individual toward active drug seeking and drug taking.
In the experiment, Dr. Regina Carelli and colleagues at the University of North Carolina at Chapel Hill (UNC-CH) exposed male lab rats to cocaine. After the rats demonstrated that they found the drug desirable--by voluntarily pressing a lever to receive doses--the researchers made a series of moment-to-moment measurements of dopamine levels in the animals' NAc. The researchers' key observation was a pair of dopamine spikes before the animals pressed the lever. The first occurred 8 seconds before the lever press; the second began 3 seconds later and peaked roughly 2 seconds after the lever press and drug delivery.
"Just the anticipation of receiving cocaine appears to cause significant increases in dopamine levels, suggesting that dopamine plays a much more complex role in addiction than simply triggering a drug's pharmacological reward," says Dr. Carelli. "Dopamine seems to alert the brain to the availability of cocaine and precipitate drug-seeking behavior. In the rats, this early spike in dopamine appears to cause them to seek the lever. In humans, the same thing may happen as part of the powerful urge to obtain drugs," she says.
Pressing the lever also triggered an audiovisual cue (light and a harmonic tone) that the rats learned to associate with the delivery of cocaine. The researchers also found that dopamine levels increased when the rats were exposed at random intervals only to the cue, in the absence of the lever and delivery of cocaine. "The dopamine activity we found in response to the cue may parallel what happens in humans who are addicted to drugs, who experience intense craving when they see drug paraphernalia or other environmental cues," Dr. Carelli observes.
To confirm the hypothesis that increases in dopamine alert rats that cocaine is available and direct their behavior toward finding the drug, the scientists used electrical stimulation to trigger dopamine release in the NAc. "In animals that had learned to self-administer cocaine, this stimulation was immediately followed by the behaviors that had preceded a lever press in the earlier sessions. The rats stopped whatever activity they were engaged in, began moving inquisitively around the chamber, and finally approached the lever," Dr. Carelli explains. "This is powerful evidence that the increase in NAc dopamine has a pivotal role in drug-seeking behavior."
To make their observations possible, the investigators used a technique called fast-scan cyclic voltammetry (see textbox). This technique lets researchers measure dopamine levels 10 times a second--hundreds of times faster than other techniques, which are limited to measurement of minute-by-minute changes.
"No one had a way to detect dopamine changes on such a fast timescale before," Dr. Carelli explains. "We are able to monitor them almost as soon as they occur. It's like listening in on the communication between brain cells as it is happening."
"These findings reveal for the first time that rapid dopamine transmission occurs during key components of cocaine-seeking behavior, during presentation of cocaine-associated cues," observes Dr. Susan Volman of NIDA's Division of Neuroscience and Behavioral Research. "The extraordinary technology used in this research allows us to detect changes in the nucleus accumbens as they happen, to observe dopamine signaling that results from a learned association between a stimulus and the drug. This adds important levels of detail to the picture of how drugs alter behavior in ways that are so destructive yet difficult to change."
Source
- Phillips, P.E.M.; Stuber, G.D.; Heien, M.L.A.V.; Wightman, R.M.; and Carelli, R.M. Subsecond dopamine release promotes cocaine seeking. Nature 422(6932):614-618, 2003. [Abstract]
Microscopic Probe Detects Changes in Brain Chemistry as They Occur
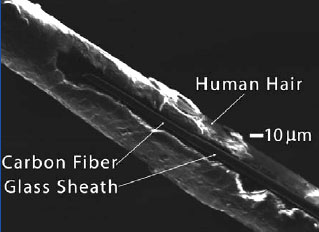 Carbon Fiber Microelectrode
Carbon Fiber MicroelectrodeFast scan cyclic voltammetry (FSCV) detects changes in electrical current that result from chemical changes in molecules deep within the brain. The technique was developed by Dr. Mark Wightman, professor of chemistry at the University of North Carolina at Chapel Hill (UNC-CH). The key to FSCV is a microscopic probe roughly 10 millionths of a meter (10 ?m) in diameter--one-tenth the thickness of a human hair (see image, right). The probe consists of a carbon fiber partially sheathed in an insulating material and connected to instruments that control the electrical properties of the fiber and record changes in current at the probe tip.
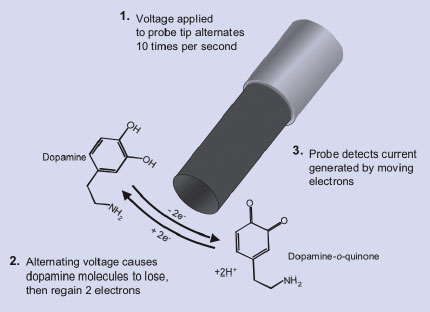
To explore the role of the neurotransmitter dopamine in rats during drug-seeking behavior, UNC-CH investigators inserted FSCV probes into the rats' nucleus accumbens. The probes are cycled through alternating positive and negative voltage (see image, below). Dopamine molecules in the region of the probe tip are oxidized (give off two electrons) when the probe has a positive voltage and reduced (retrieve two electrons) when the probe's charge shifts to negative. The transfer of electrons generates an electric current at the probe surface that is recorded by instruments attached to it. The voltage at the probe's tip can be reversed rapidly, and FSCV is able to measure current changes--whose magnitude directly reflects changes in dopamine concentration--at intervals as short as 10 times per second. The measurements made with FSCV in the UNC-CH dopamine studies are the fastest measurements of chemical change ever recorded in active living animals, the researchers say.
In 2001, NIDA awarded Dr. Wightman and UNC-CH colleague Dr. Regina Carelli a Cutting Edge Basic Research Award (CEBRA) grant to explore the potential of FSCV in drug abuse research. "CEBRA grants are designed to support research that is high-risk and potentially high-impact, and the first results of NIDA's support of this technology are impressive," explains Dr. Nancy Pilotte of NIDA's Division of Neuroscience and Behavioral Research.
The next stage of the UNC-CH scientists' dopamine research will simultaneously monitor dopamine-producing cells as they release the neurotransmitter and the electrical changes in dopamine-receiving cells as they respond to it." Measuring neurotransmitter and electrical activity as they occur may make possible the creation of a real-time map of neurons responding to changing dopamine concentrations," Dr. Pilotte says. "We could see brain cells self-assembling circuits in response to a stimulus. It would be like watching learning as it happens."
