The scientific view of drug addiction as a chronic brain disease rests on many studies showing that addictive drugs change the brain in ways that cause compulsive drug seeking and drug taking. NIDA research recently demonstrated that the converse can also sometimes be true: In rats, behavioral modification changed the biochemistry of the brain and thereby reduced the motivation to self-administer cocaine.
Dr. David W. Self of the University of Texas Southwestern Medical Center in Dallas and colleagues at the Yale University School of Medicine and Harvard Medical School induced cocaine dependency in a set of experimental animals and then examined the impact of the behavioral technique called "extinction training" on the rats' behavior and glutamate receptors in one of the brain's major communications systems. Their finding that the training increased quantities of the glutamate receptors underscores the potential effectiveness of behavioral treatments for addiction and relapse prevention.
Extinction Training and Glutamate
The researchers began their experiments by training rats to self-administer cocaine at will by pressing a lever. Animals trained in this way become "cocaine dependent"—that is, they develop behavioral and neurobiological changes that simulate the effects of addiction in people. The rats then underwent extinction training. In this technique, the researchers put animals into cages where they have self-administered a drug, but with a difference: Now, when the animals press the lever, no drug is dispensed. After a number of tries—more for some, less for others—the animals lose interest in the lever. Basically, extinction training reeducates animals, teaching them that the association between pressing the lever and getting a drug no longer holds.
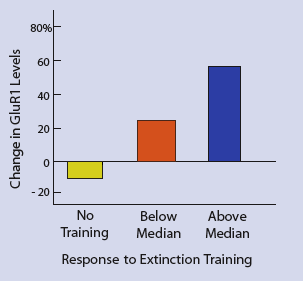 Stronger Training Response Correlates With Higher Receptor Levels. Rats that responded more strongly to extinction training showed greater increases in glutamate receptor levels. Rats that did not receive training demonstrated a decrease in glutamate receptors. Percent changes shown are relative to control rats not exposed to cocaine.
Stronger Training Response Correlates With Higher Receptor Levels. Rats that responded more strongly to extinction training showed greater increases in glutamate receptor levels. Rats that did not receive training demonstrated a decrease in glutamate receptors. Percent changes shown are relative to control rats not exposed to cocaine.In their first experiment, the researchers measured how extinction training affected the frequency of the cocaine-dependent rats' lever pressing and the supply of two glutamate receptors in the part of the brain known as the shell of the nucleus accumbens (NAc). The receptors, GluR1 and GluR2, act as relays in the brain's glutamate system, which uses the neurotransmitter glutamate to send messages from cell to cell.
As expected, extinction training changed the rats' behavior: Among the rats that received it, the frequency of lever pressing declined from a range of 39-44 presses in a 4-hour interval to a rate equivalent to 22-34 presses. These rats were also found to have 39% more GluR1 and 31% more GluR2 than matched cocaine-dependent rats not subjected to extinction training. Strengthening the conclusion that extinction training was responsible for these increases, the sizes of the declines in lever pressing correlated with the amount of increase in GluR1. Rats whose declines exceeded the median averaged a 58% increase in GluR1, while those whose declines were less than the median averaged a 24% increase.
"We discovered something profound—that simply allowing an animal to press a lever and not get any drug completely changed the brain's response to cocaine," Dr. Self says.
A second experiment confirmed the relationship between the glutamate receptors and successful extinction training. It also showed that the relationship goes the other way, too: Having more receptors at the time of extinction training increases the response to the training. In preparation for this experiment, the researchers used genetic engineering to artificially increase GluR1 and GluR2 in some cocaine-dependent rats' NAc's. In the first hour of training, these animals pressed levers for cocaine roughly 20 times, while animals whose receptors were not enhanced performed about 40 lever presses. The animals with the extra glutamate receptors also achieved the milestone of fewer than 20 lever presses after only 2 to 2.5 days of training, compared with more than 4 days for the other animals.
Summarizing the results of the two experiments, Dr. Self says, "There is a reciprocal relationship between extinction training and the level of glutamate receptors. Extinction training causes transient increases in glutamate receptors, while higher quantities of glutamate receptors facilitate extinction of drug-seeking behavior."
To explain why rats with enhanced glutamate receptors are more likely to pass up cocaine, Dr. Self points to one of the key functions of the glutamate system: It helps enable an animal or a person forego short-term pleasures for the sake of safety or longer-term goals. In this situation, the prefrontal cortex, the brain's seat of foresight and planning, releases glutamate molecules into the NAc, the brain's pleasure center, that override the motivation for the short-term pleasure. Previous research has shown that chronic cocaine exposure reduces the cortex's glutamate production, weakening its influence over the pleasure center. Dr. Self suggests that increasing the number of glutamate receptors in the NAc may partially compensate for the glutamate shortage by relaying more signals from the glutamate that still remains.
A more difficult question—one the researchers are pursuing now—is why extinction training increased glutamate receptors in the NAc. Although cocaine also caused glutamate shortages in rats that were taken off it without extinction training, those animals did not respond with compensatory increases in receptors.
Stress and Relapse
In a subsequent experiment, Dr. Self and his colleagues demonstrated that raising GluR1 levels in the NAc reduced formerly dependent rats' tendency to respond to stress by reverting to cocaine self-administration. Most surprising, the reduction in response was documented 3 weeks after the elevated GluR1 levels returned to normal.
After inducing cocaine dependency, genetically increasing the supply of glutamate receptors in the NAc, and a single session of extinction training, the researchers gave their experimental rats a 3-week timeout. Then, they returned the rats to their training cages and administered three types of stimulus: a "priming" dose of cocaine, a light that flashes when the lever in the cage is ready to dispense cocaine, and a series of mild electrical shocks to the paw. These stimuli correspond to triggers that commonly cause addicted people to relapse—drug exposure, environmental cues associated with previous drug use, and stress. Each usually causes rats to revert to drug self-administration. Dr. Self and his colleagues found, however, that when their extinction-trained rats received foot shocks, those whose GluR1 levels were higher resumed lever pressing about 87% less frequently than those with less GluR1.
"This was really surprising," says Dr. Self. "It suggested that glutamate receptor increases during extinction training have long-term effects on stress-induced relapse, even after receptor quantities return to normal."
To account for this finding, Dr. Self suggests that "there is a similarity between stress and extinction training. Foot shock is mild and basically irritates the animal, the rat equivalent of a bad day at work. Extinction is stressful too—the frustration of looking for a drug you anticipate but don't get. Perhaps extinction training teaches the animal to cope with stress by inhibiting craving, which stress normally would increase."
Dr. Nancy S. Pilotte of NIDA's Division of Neuroscience and Behavioral Research agrees that behavioral changes sustained beyond the transient period of glutamate receptor elevation are an important finding. "This tells you it is extremely important to follow animals after drugs are withdrawn, a stage of addiction we know fairly little about," she says. "We know a lot about acute affects, but very little about what happens to animals when they are no longer receiving drugs."
Currently, no equivalent to extinction training exists for treating people. However, Dr. Self believes that in the future, virtual reality technology may make such an approach possible. "A very realistic video game might make it possible to recreate many of the anticipatory and emotional responses involved in preparing for the drug experience without ever actually receiving the drug," he explains. "Repeated 'virtual drug taking' could extinguish these emotional responses by strengthening the brain pathways that exert inhibitory behavioral control over drug craving."
Source
- Sutton, M.A.; Schmidt, E.F.; Choi, K-H.; Schad, C.A.; Whisler, K.; Simmons, D.; Karanian, D.A.; Monteggia, L.M.; Neve, R.L.; and Self, D.W. Extinction-induced upregulation in AMPA receptors reduces cocaineseeking behaviour. Nature421(6918):70-75, 2003. [Abstract]
