Using genetic engineering, NIDA-supported scientists have produced a strain of mice with special characteristics that can help researchers identify and study key steps in the development of nicotine addiction. By altering a single amino acid in just one of a mouse's 30,000 genes, the scientists produced mice that are exceptionally sensitive to the effects of nicotine. The modified mice show behaviors associated with addiction when exposed to nicotine doses far too small to cause similar effects in other mice. Their dramatically increased sensitivity suggests that the brain cell site affected by the modified gene is crucial to development of nicotine addiction.
Dr. Andrew Tapper and colleagues at the California Institute of Technology in Pasadena and at the University of Colorado in Boulder built on work by other scientists which indicated that a site on some brain cells—the α4 subunit of nicotine receptors—plays a key role in the brain's response to nicotine. The previous work involved "knock-out" mice, in which scientists had disabled a gene that directs development of the α4 site. When exposed to nicotine, the α4 knock-out mice did not respond with increased release of the pleasure-causing brain chemical dopamine, a reaction thought to be a key factor in the development of nicotine addiction.
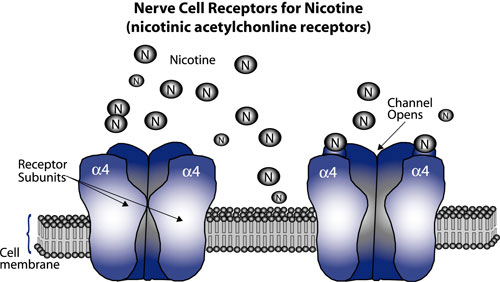 Brain Pathway to Nicotine Addiction. Nicotine attaches to nerve cells in the brain at receptors on the cell membrane. The receptors comprise five subunits that fit together like sections of an orange. When a nicotine molecule binds to one of these subunits, the segments pull away from each other, creating an open channel through the cell membrane. This initiates a series of electrical and chemical signals that trigger release of dopamine by other brain cells. One type of subunit, designated α4, appears to play a central role in development of nicotine addiction; mice engineered to have especially sensitive α4 subunits exhibit behaviors characteristic of nicotine addiction when exposed to a dose of nicotine just one-fiftieth of that normally needed to elicit these behaviors.
Brain Pathway to Nicotine Addiction. Nicotine attaches to nerve cells in the brain at receptors on the cell membrane. The receptors comprise five subunits that fit together like sections of an orange. When a nicotine molecule binds to one of these subunits, the segments pull away from each other, creating an open channel through the cell membrane. This initiates a series of electrical and chemical signals that trigger release of dopamine by other brain cells. One type of subunit, designated α4, appears to play a central role in development of nicotine addiction; mice engineered to have especially sensitive α4 subunits exhibit behaviors characteristic of nicotine addiction when exposed to a dose of nicotine just one-fiftieth of that normally needed to elicit these behaviors.The results with knock-out mice suggested that α4 sites on brain cells are necessary for development of nicotine addiction, but didn't address the question of whether the sites are sufficient by themselves to initiate the behaviors associated with addiction. To answer that question, says Dr. Henry Lester of the California Institute of Technology, "We decided to create animals with hypersensitive α4 receptors. That way, instead of eliminating the response to nicotine, we could emphasize it and study the processes that lead to nicotine addiction. So we developed the α4 'knock-in' mouse."
The scientists compared the behavioral effects that are in part characteristic of nicotine addiction—reward, tolerance, and sensitization—in their knock-in mice and unmodified mice. According to Dr. Lester, the results indicate that activation of the α4 site by nicotine is sufficient to initiate the effects.
Reward: The researchers measured nicotine reward in their mice with a technique called "conditioned place preference," which is based on the assumption that if animals like an experience, such as receiving nicotine, they will gravitate to the place where they have had that experience rather than another where they haven't. In the experiment, mice with unmodified α4 receptors exhibited a preference for a compartment associated with a nicotine dose of 0.5 mg/kg of weight—a typical dose ingested by a human smoker. The investigators then tested the rewarding effect of one-fiftieth of that amount, 10 μg/kg, on the unmodified and the α4 knock-in mice. When allowed to move freely between the chambers for 20 minutes following nicotine administration, the unmodified mice showed no preference for the nicotine-associated compartment; they spent slightly less time in that chamber than they had before. In contrast, modified mice showed a marked preference for the compartment associated with nicotine, spending an average of 2 minutes more in that chamber following nicotine administration.
Tolerance and sensitization: To test tolerance to nicotine, the investigators subjected the unmodified and knock-in mice to repeated doses of nicotine, 15 μg/kg daily over 9 days, and then compared the changes in nicotine-induced hypothermia. The unmodified mice showed no change in temperature, but the knock-in mice exhibited a decrease of 3°C on the first and second days, and smaller decreases each successive day, suggesting they had developed tolerance to the nicotine-induced hypothermia. In tests for sensitization, only the genetically engineered mice increased activity levels (measured by counting the number of times the animals cross a beam of light in the 60 minutes following injection) in response to daily injections of 15 μg/kg over 9 days.
"This work represents a significant step forward in understanding how nicotine hijacks the brain's normal signaling process," says Dr. Joni Rutter of NIDA's Division of Basic Neuroscience and Behavioral Research. "And the research approach—moving from manipulation of a single protein to an animal's behavioral response to nicotine—also holds great promise. If the α4 site is also found to play a large role in human nicotine addiction," Dr. Rutter adds, "it is a promising focus for research into medications that might block nicotine's effects."
Source
- Tapper, A.R., et al. Nicotine activation of α4 receptors: Sufficient for reward, tolerance, and sensitization. Science 306(5698):1029-1032, 2004. [Abstract]
Genetic Engineering Reveals Proteins' Key Role in Sensitivity to Cocaine
Genetic engineering strategies like those used at the California Institute of Technology to study nicotine addiction have helped other investigators identify a pair of proteins that seem to influence cocaine addiction.
Dr. Peter Kalivas and his colleagues at the Medical University of South Carolina in Charleston developed a strain of mice lacking two genes, called Homer1 and Homer2, that direct production of proteins linked to cocaine's effects in the brain. The researchers found that the Homer "knock-out" mice were more sensitive than unmodified mice to the behavioral effects of cocaine.
Compared with unmodified mice, animals missing either Homer1 or Homer2 developed stronger place conditioning—when allowed to move freely, they would spend more time in a compartment where they had received cocaine than in a compartment with no drug association. The knock-out mice also were more sensitive to cocaine's stimulatory effect; when placed in a chamber equipped with photoelectric beams that could measure activity, the knock-outs were approximately 50 percent more active than unmodified mice following cocaine injections. To verify the role of the Homer genes in increased sensitivity to cocaine, the researchers restored Homer genes in the brains of the knock-outs, eliminating the previously seen differences in stimulation and place conditioning.
"The fact that Homer deletions result in these augmented responses to cocaine suggests that disruption of Homer protein-regulated signaling in the brain is a central step in development of cocaine addiction," Dr. Kalivas says. Additional evidence of this role is seen in changes that Homer deletion causes in levels of the brain messenger chemical glutamate, he adds. Homer knock-out mice that had never been exposed to cocaine had nucleus accumbens (NAc) glutamate concentrations about 50 percent lower than mice with the genes—an effect similar to that seen in mice after cocaine withdrawal. This effect, too, was reversed when the scientists injected Homer genes into the NAc.
The association between Homer activity and the conditions of cocaine withdrawal is particularly intriguing, according to Dr. Kalivas, because other researchers have shown that Homer protein levels rise and fall in response to environmental cues and changing levels of stress. "Homer may be a window to study the molecular basis of the important link between environmental stress and cocaine addiction."
Source
- Szumlinski, K.K., et al. Homer proteins regulate sensitivity to cocaine. Neuron 43(3):401-413, 2004. [Abstract]
