Baboons that are chronically exposed to gamma-hydroxybutyrate (GHB), a club drug known as "liquid ecstasy" and "Georgia home boy," develop withdrawal symptoms when drug administration stops, NIDA-supported investigators have shown. The animals' symptoms—tremors, loss of appetite, and agitation—are similar to those that occur when GHB-dependent people stop taking the drug. The finding, the first clear demonstration of GHB withdrawal in an animal, will enable researchers to use baboons as subjects in studies of the drug's effects in the brain and possible pharmacological treatments for withdrawal. Additional findings bolstered evidence from previous research suggesting that GHB dependence may be largely due to the drug's action at sites where the chemical messenger gamma-aminobutyric acid (GABA) attaches to brain cells.
"It is important to be able to study GHB's effect in another species because human studies involving high doses are too risky," says Dr. Minda Lynch of NIDA's Division of Basic Neuroscience and Behavioral Research. "Chronic GHB abusers report severe withdrawal symptoms, which make it very difficult for them to quit abusing the drug. A slight increase in dose can tip GHB's sedative effects to a lethal level, and those who continue abusing the drug because withdrawal is so hard to bear are at risk for possibly fatal effects."
Dr. Elise Weerts and her colleagues at The Johns Hopkins University conducted a three-part study to evaluate GHB withdrawal in baboons. They observed the behavioral effects of GHB administered daily for 1 month, tried to precipitate withdrawal after the baboons had been receiving GHB for 2 weeks by treating the animals with a compound that affects brain sites called GABA-B receptors, and discontinued GHB administration after 4 weeks to see if the baboons experienced withdrawal.
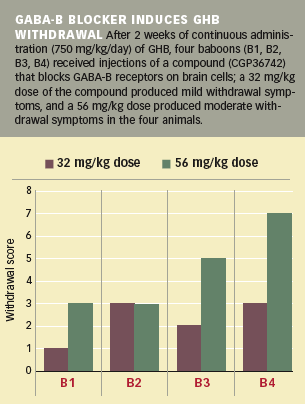
During the first 2 weeks of daily GHB administration (750 mg/kg of body weight) the animals showed behavioral changes that confirmed to the researchers that GHB was having neurobiological effects at the dosage used; they spent more time in resting postures and had difficulty with tasks requiring fine motor skills, such as picking raisins or M&Ms from small cups. After 2 weeks of GHB administration, each baboon received injections (32 and 56 mg/kg, 8 days apart) of a compound that blocks GHB from attaching to GABA-B receptors—structures composed of proteins that influence brain cells' responses to the chemical GABA, one of the brain's primary carriers of intercellular communications. Immediately after injection of the GABA-B blocker, the baboons exhibited symptoms that included tremors, vomiting, and increased aggression, together with milder behavioral changes, such as assuming abnormal postures and sitting with their eyes closed. These symptoms increased with higher doses of the GABA-B blocker and dissipated after its effects wore off. When the researchers discontinued GHB administration after 1 month, the animals exhibited the same withdrawal symptoms as those triggered by the GABA-B blocker.
"The daily dose these animals received was equivalent to doses reportedly abused by humans and thought to produce dependence. The spontaneous withdrawal that occurred when GHB administration was discontinued and the withdrawal precipitated by the GABA-B blocker are evidence that the baboons were physically dependent on GHB," Dr. Weerts concludes. Moreover, the observed effects of the GABA-B blocker reinforce earlier findings that GHB acts at these receptors, Dr. Weerts says, as well as the suggestion that these sites are possible targets for therapies to counteract or inhibit GHB's effects. Dr. Weerts next plans to use her baboon model to try to quantify the combination of GHB dosage and duration of exposure that leads to dependence and to test other drugs that may precipitate or alleviate withdrawal.
Sources
- Weerts, E.M., et al. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology179(3):678-687, 2005.
- Goodwin, A.K.; Froestl, W.; and Weerts, E.M. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology 180(2):342-351, 2005.