Heroin-addicted patients who undergo so-called ultrarapid, anesthesia-assisted detoxification suffer withdrawal symptoms as severe as those endured by patients in detoxification by traditional methods, according to a NIDA-funded clinical trial. Researchers Dr. Eric Collins and colleagues at the College of Physicians and Surgeons of Columbia University concluded that there is no compelling reason to use general anesthesia in the treatment of opiate dependence, especially as it presents particular safety concerns. The new findings corroborate those of three international studies.
The ultrarapid detox technique, developed about 15 years ago by clinicians who hoped to mitigate the discomfort of withdrawal and speed the initiation of relapse prevention therapy, relies on a general anesthetic to sedate the patient for several hours while an opiate blocker precipitates withdrawal.The method is not covered by insurance, which makes it difficult to determine how many patients have received anesthesia-assisted detox.
To compare anesthesia-assisted detox with other approaches, Dr. Collins and colleagues enrolled 106 people seeking heroin detox at Columbia University Medical Center's Clinical Research Center. The patients, aged 21 through 50, had abused heroin every day during the past month. All spent 3 days as Center inpatients during detox, then were scheduled for twice-weekly outpatient relapse prevention psychotherapy and naltrexone maintenance (50 mg/day) for 12 weeks.
The investigators randomly assigned the participants to one of three detox methods (see chart). The goal of each method was to minimize patients' discomfort during withdrawal. In the ultrarapid approach, physicians put patients under anesthesia for 4 to 6 hours while administering naltrexone, a medication that precipitates withdrawal by blocking opioid molecules from their receptors in the brain. In the second method, patients remained awake and took a single dose of buprenorphine, a medication that eases withdrawal symptoms by moderating and smoothing the rate of opioid clearance from the brain. In the third approach, patients also remained awake and received clonidine and other nonopioid medications as needed to counter symptoms for all 3 inpatient days. These medications were available to all groups as needed for the duration of the inpatient phase. Throughout detox, the researchers closely monitored patients for complications, assessed physical indications of withdrawal, and asked the participants to rate their subjective experiences.
| Opiate Detox Methods | Inpatient treatment | Outpatient treatment | |||
|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 through week 12 | |
| Anesthesia- Assisted | Anesthesia 4-6 h → 2 h monitoring in post-anesthesia unit → naltrexone induction (50 mg) Clonidine and nonopioid medications as needed for with-drawal symptoms | Begin naltrexone maint-enance (50 mg/day) (continue through end of study) Ancillary withdrawal medications continued | Discharge from inpatient treatment Ancillary withdrawal medications continued | Twice-weekly psycho-therapy Naltrexone maint-enance medication (50 mg/day) | |
| Bupren- orphine- Assisted | Bupren-orphine (8 mg) | Clonidine and nonopioid medications as needed for with-drawal symptoms | Naltrexone induction (12.5 mg) Ancillary withdrawal medications continued | Discharge from inpatient treatment Naltrexone induction continues (25 mg) Ancillary withdrawal medications continued | Twice-weekly psycho-therapy Naltrexone maint-enance medication (50 mg/day) |
| Clonidine- Assisted | Clonidine and nonopioid medications as needed for with-drawal symptoms | Ancillary withdrawal medications continued | Ancillary with-drawal medications continued Discharge from inpatient treatment | Twice-weekly psycho-therapy Begin 2-day naltrexone induction on day 7 (12.5 mg, then 25 mg), followed by naltrexone maint-enance starting on day 9 (50 mg/day) | |
Once awakened from anesthesia, patients in the ultrarapid detox group demonstrated and reported symptoms of discomfort comparable to those experienced by participants receiving the buprenorphine- and clonidine-assisted methods (see chart). Three patients receiving the anesthesia-assisted method experienced serious adverse events—pulmonary and psychiatric complications as well as a metabolic complication of diabetes, all of which required hospitalization. The complications were related to preexisting medical conditions that the patients had failed to reveal when they were screened for admission into the study. No adverse events occurred with the other detox methods.
Treatment outcomes among the three groups were similar. Following detox, the researchers offered all the patients relapse prevention therapy consisting of outpatient counseling and naltrexone, which counteracts the pleasurable effects of subsequently administered opioids. More than 90 percent of the patients who received the anesthesia- and buprenorphine-assisted detox completed naltrexone induction; only 21 percent of those receiving clonidine completed induction. By the third week, more than half the patients in all three groups had dropped out of the study; only 18 percent remained in treatment the full 12 weeks. The percentages of patients submitting opiatepositive urine samples during outpatient treatment also were comparable, roughly 63 percent, across the three detox methods.
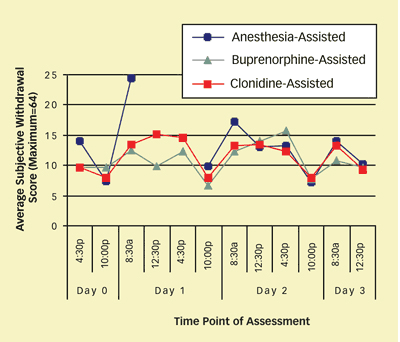 In Three Detox Methods, Withdrawal Symptom Severity Was Similar: During a 72-hour inpatient detoxification stay, patients rated each of 16 withdrawal symptoms—for example, "I feel like vomiting," "I have cramps in my stomach," "I feel anxious," and "My eyes are tearing"—on a scale from 0 (not at all) to 4 (extremely). Symptom severity generally did not differ between heroin-addicted patients receiving anesthesia-, buprenorphine-, or clonidine-assisted methods. Researchers did not assess withdrawal symptoms for the anesthesia-assisted group during general anesthesia and the immediate recovery period.
In Three Detox Methods, Withdrawal Symptom Severity Was Similar: During a 72-hour inpatient detoxification stay, patients rated each of 16 withdrawal symptoms—for example, "I feel like vomiting," "I have cramps in my stomach," "I feel anxious," and "My eyes are tearing"—on a scale from 0 (not at all) to 4 (extremely). Symptom severity generally did not differ between heroin-addicted patients receiving anesthesia-, buprenorphine-, or clonidine-assisted methods. Researchers did not assess withdrawal symptoms for the anesthesia-assisted group during general anesthesia and the immediate recovery period."No Advantage"
"Although providers advertise anesthesia-assisted detox as a fast and painless method to kick opiate addiction, the evidence does not support those statements," says Dr. Collins. "Patients should consider the many risks associated with this approach, including fluid accumulation in the lungs, metabolic complications of diabetes, and a worsening of underlying bipolar illness, as well as other potentially serious adverse events," he says. Those with preexisting medical conditions—including some psychiatric disorders, elevated blood sugar, insulin-dependent diabetes, prior pneumonias, hepatitis, heart disease, and AIDS—are particularly at risk for anesthesia-related adverse events. "Careful screening is essential with the anesthesia-assisted method, because the thought of sleeping through withdrawal is so compelling that some patients may conceal their medical histories," says Dr. Collins.
"We now have several rigorous studies indicating that anesthesia-assisted detox— a costly and risky approach—offers no advantage over other methods," says Dr. Ivan Montoya of NIDA's Division of Pharmacotherapies and Medical Consequences of Drug Abuse. Dr. Montoya notes, "The low retention of patients in subsequent outpatient treatment in the present study, which is not unusual for the opiate-addicted population, highlights the need to engage people in long-term recovery after detoxification." Naltrexone can help motivated patients stay off opiates, but many do not stick to the regimen of daily tablets because of the medication's side effects of anxiety and restlessness. Long-acting monthly injections of naltrexone, which are now available for alcoholism treatment, may work better for patients and show promise in NIDA-supported clinical trials.
Dr. Montoya also points out that with the current epidemic of prescription painkiller abuse, clinicians need more research on costeffective detox methods for these opiates (see "2003 Survey Reveals Increase in Prescription Drug Abuse, Sharp Drop in Abuse of Hallucinogens"). Some clinics are using buprenorphine for this purpose, and NIDA-funded investigators are studying various methods to improve prescription opiate detox and help patients engage in longer term treatment.
Source
Collins, E.D., et al. Anesthesia-assisted vs buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: A randomized trial.Journal of the American Medical Association 294(8):903-913, 2005. [Abstract]
