Most people who become chronic smokers start in adolescence, and the risk of addiction at this time is even greater among those whose mothers smoked while pregnant. NIDA-funded animal studies recently identified two neurobiological effects of nicotine that could underlie these vulnerabilities. Investigators at the University of Tennessee, led by Dr. Burt Sharp, found that prenatal nicotine exposure reduces the availability during adolescence of a receptor that mediates the drug's impact on cells in the brain's reward system. At the University of Wisconsin, Dr. Charles Landry and his research team found that nicotine stimulates a set of genes involved in synapse formation to a higher level of activity in adolescent than in adult rats.
Nicotine's Impact on Receptors
The University of Tennessee researchers pursued a clue from previous work in which they examined the effects of prenatal nicotine exposure on the mesolimbic reward pathway. Nicotine and other drugs of abuse stimulate neurons in the brain area where this pathway originates, the ventral tegmental area (VTA), to release the neurotransmitter dopamine in the nucleus accumbens (NAc) and prefrontal cortex (PFC). The dopamine influx into the NAc produces the feelings of reward and pleasure that are primary motivators of continued drug-taking. Dr. Sharp and colleagues found, however, that exposing rats prenatally to nicotine reduced the amount of dopamine released in the NAc when the animals were given the drug again as adolescents.
| Brain Region | Male | Female | ||
|---|---|---|---|---|
| Control | Nicotine | Control | Nicotine | |
| NAc | 38.2 | 30.2 | 37.7 | 27.1 |
| PFC | 55.1 | 45.6 | 55.7 | 41.9 |
| VTA | 59.5 | 43.3 | 45.9 | 39.3 |
"We asked ourselves, 'What causes this?'" Dr. Sharp says. "We decided to look at nicotine's impact on the expression of nicotinic cholinergic receptors—the principal sites where nicotine molecules interact with brain cells to exert their stimulating effects." The researchers hypothesized that exposure to nicotine during gestation would reduce the number of such receptors present on dopamine-producing cells in the VTA in adolescence.
They gave nicotine to pregnant rats via an implanted pump at the rate of 2 mg/kg/day (the equivalent of a human smoking a pack a day) throughout gestation. At birth they increased the nicotine infusions to 6 mg/kg/day and continued them for 2 more weeks, while the rat pups nursed. Because rat pups are born at an earlier stage of development than humans, the weeks of continued exposure were necessary to give them cumulative nicotine exposure equivalent to a smoking mother's baby at full-term. A control group of rats received the nicotine delivery solution without the drug.
The researchers took brain sections from the rat pups when they were 35 days old, developmentally equivalent to mid-adolescence in humans, and assayed them for nicotinic cholinergic receptors. In confirmation of their hypothesis, the results showed significantly fewer receptors in the VTA, NAc, and PFC of the adolescent rats that had been exposed to nicotine in utero. Messenger RNA (mRNA) for the receptors declined only in the VTA, suggesting that gestational nicotine had primarily affected dopaminergic neurons that originate in that area. The total number of VTA neurons also dropped in the brains of nicotine-exposed rats.
These findings "show how gestational exposure to nicotine may alter maturation, literally changing the brain," Dr. Sharp says. Although it is not clear how such changes could enhance the likelihood of dependence, "one hypothesis might be that prenatally exposed adolescents, having fewer nicotinic receptors, must take more puffs to release a rewarding amount of dopamine into the NAc, and this leads to stronger conditioning," he says. Dr. Allison Chausmer of NIDA's Division of Basic Neuroscience and Behavioral Research says, "The findings confirm the long-term effect of smoking during pregnancy and underscore the importance of smoking cessation at this time."
Nicotine Affects Synapse Development
The University of Wisconsin team studied the impact of nicotine on genes that contribute to neural plasticity. This process—the formation of new synaptic connections between neurons and pruning of old ones—wires the brain during development and reaches a crescendo during adolescence. The researchers specifically focused on the genes—including arc, c-fos, and NGFI-B—that produce a set of neurochemicals involved in building synapses. Using rats as subjects, they compared the expression—roughly, the production rate—of these genes following exposure to nicotine in adolescents (average age 30 days) and adults (average age 70 days).
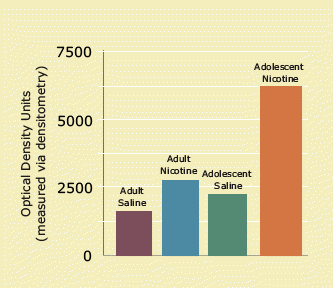 arc Expression Increases With Nicotine: The height of the bars represents the expression of arc, a gene involved in neural plasticity. Administration of nicotine increases arc in the ventral and lateral cortex of both adolescent and adult rats, but significantly more in adolescents. This suggests that nicotine triggers synaptic development—a key process in learning—in a region important in motivation and goal-directed activity. Because the effect is greater in adolescents, they may more readily "learn" the nicotine habit.
arc Expression Increases With Nicotine: The height of the bars represents the expression of arc, a gene involved in neural plasticity. Administration of nicotine increases arc in the ventral and lateral cortex of both adolescent and adult rats, but significantly more in adolescents. This suggests that nicotine triggers synaptic development—a key process in learning—in a region important in motivation and goal-directed activity. Because the effect is greater in adolescents, they may more readily "learn" the nicotine habit.The investigators injected the rats with nicotine at a dose large enough (0.4 mg/kg) to cause a behavioral response—increased motor activity—or with saline. An hour later, they examined slices of the rats' brains, with particular attention to areas that play central roles in learning and motivation: the medial PFC, ventral and lateral orbital cortex (VLO), cingulate cortex, somatosensory cortex, ventral striatum, and dorsal striatum. They assessed the expression of plasticity-related genes by measuring the amount of their corresponding mRNA.
Throughout the brain, they found higher amounts of mRNA for arc and c-fos in the adolescent than the adult brains, an indication of more synaptic plasticity overall, Dr. Terri Schochet suggests. In both age groups, arc and c-fos mRNA jumped after injection of nicotine, compared with saline, indicating that the drug "switched on" these genes. In certain prefrontal regions, the nicotine-evoked increase in arc mRNA was significantly greater in adolescent animals. In the VLO, for example, arc expression increased by 182 percent in adolescents after nicotine injection, compared with 98 percent in adults.
"These findings show that at the basic biochemical level, the adolescent brain responds differently to a single dose of nicotine," says Dr. Landry, principal investigator for this study. "The enhanced expression of arc, a gene involved in dendrite formation, in adolescent forebrains following acute nicotine reflects a very dynamic synaptic milieu. It's difficult to speculate further, but my suspicion is that the adolescent brain responds to the drug with a greater increase in synaptogenesis and pruning."
"The adolescents' greater changes in molecular systems involved in learning may indicate that this age group is more susceptible to developing the nicotine habit," Dr. Schochet suggests. The striking effect of a single dose of nicotine could have implications for treatment, she adds: "It's really important to intervene as early as possible to prevent adolescents from trying nicotine in the first place."
Research that explores and compares adult and adolescent behavior and neurobiology is a particular interest of NIDA's, says Dr. Susan Volman of the Institute's Division of Basic Neuroscience and Behavioral Research. This study was valuable because it "looks at both what's different in general between the maturing and adult brain and how that difference interacts with nicotine."
Dr. Volman notes that the adult/adolescent disparity in response to nicotine was greatest in the ventrolateral PFC. "Neural adaptations here could have to do with altering motivation and the value placed on particular rewards," she says. Smoking might be equally pleasurable to adults and adolescents, that is, but the experience would be more highly valued by the adolescent—a difference with potential implications for tailoring behavioral treatments to this age group.
Sources
Chen, H., et al. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. European Journal of Neuroscience 22(2):380-388, 2005. [Abstract]
Schochet, T.L., Kelley, A.E., and Landry, C.F. Differential expression of arcmRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience 135(1):285-297, 2005. [Abstract]
