In a NIDA-supported pilot study, a new formulation of naltrexone that patients receive by injection once every 30 days appeared safe and helped heroin-addicted outpatients persevere in treatment. Investigators observed a dose-dependent relationship between the medication, called depot naltrexone, and patient retention rates.
Naltrexone helps patients overcome urges to abuse opiates by blocking the drugs' euphoric effects. Some patients do well with it, but the oral formulation, the only one available to date, has a drawback: It must be taken daily, and a patient whose craving becomes overwhelming can obtain opiate euphoria simply by skipping a dose before resuming abuse.
"What's exciting about this slow-release formula is that it provides continuous protection for a month at a time, freeing patients from having to decide to take or not take the medication every day," says Dr. Sandra Comer, lead investigator of the study. "By increasing treatment retention, depot naltrexone may allow patients greater contact with appropriate supportive counseling and ease their transition to a life without heroin."
Dr. Comer and her collaborators recruited 60 heroin-addicted, predominantly male (77 percent) adults, aged 18 to 59 years, through advertising in local newspapers and word of mouth in New York City and Philadelphia. To be eligible, patients could not be addicted to any drugs other than heroin, caffeine, or nicotine. After initial heroin detoxification, the investigators randomly assigned participants to receive low-dose depot naltrexone, high-dose depot naltrexone, or placebo at the beginning of weeks 1 and 5. All participants received twice-weekly relapse prevention behavioral therapy.
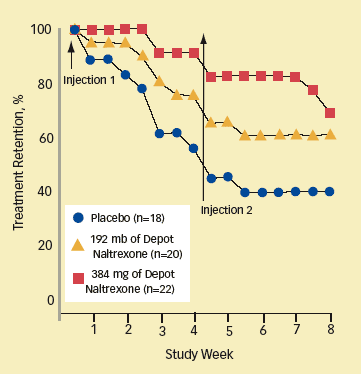 Naltrexone Helps Patients Stay in Treatment: More patients receiving 384 mg of depot naltrexone attended each weekly treatment session, compared with those receiving a smaller dosage of depot naltrexone or those who received placebo.
Naltrexone Helps Patients Stay in Treatment: More patients receiving 384 mg of depot naltrexone attended each weekly treatment session, compared with those receiving a smaller dosage of depot naltrexone or those who received placebo.After 8 weeks, 68 percent of the patients receiving 384 mg of naltrexone remained in treatment, compared to 60 percent of those receiving 192 mg, and 39 percent of those on placebo. The percentage of urine samples negative for opioids was highest for the group receiving 384 mg of naltrexone (62 percent) and lowest for the placebo group (25 percent). After receiving the medication, patients in the naltrexone groups reported "needing heroin" significantly less than those taking placebo.
The study participants experienced no apparent serious side effects. Despite previous reports associating high doses of naltrexone with hepatotoxicity, only one patient developed elevated liver enzymes, which the researchers attributed to a new-onset hepatitis C infection rather than the medication. Heroin overdose, another potential concern for patients on naltrexone, was not observed in the study; several patients did abuse heroin while on naltrexone, but reported no pleasure from it.
Encouraged by their results, Dr. Comer and her colleagues are beginning a 6-month trial with a larger number of participants. "We want to make sure the depot formula helps over a longer period of time," she explains. "Having more tools is really helpful for providers. Some people do better on methadone, others on naltrexone. We'll have more success if we can offer both."
Dr. Richard Hawks of NIDA's Division of Pharmacotherapies and Medical Consequences of Drug Abuse, says pharmaceutical companies are developing even longeracting versions of naltrexone—a 6-month sustained-release formula. "But a drug alone never works," he says. "To be effective, the medication must be combined with behavioral therapy. Many years of behavioral therapy research shows that the longer someone is in treatment, the longer the time to relapse. Longer-acting, sustained-release medications help maximize this effect."
Source
Comer, Sandra D., et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence. Archives of General Psychiatry 63(2):210-218, 2006. [Abstract]
