A naturally occurring brain chemical has shown early promise as a treatment for addiction. NIDA-funded researcher Dr. William Millington and colleagues at Albany College of Pharmacy demonstrated that glycyl-glutamine (Gly-Gln), a product of the conversion of one form of beta endorphin to another, reduces the rewarding effects of morphine and nicotine and the severity of withdrawal from these drugs in rats.
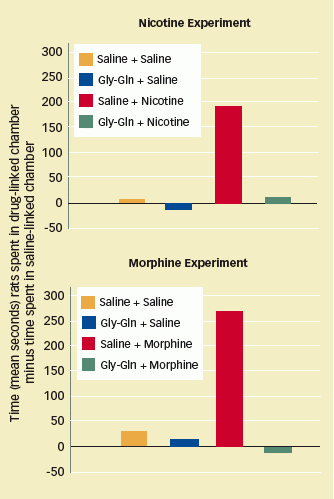 Gly-Gln Blocks the Rewarding Effects of Nicotine and Morphine: Rats trained to associate nicotine or morphine infusions with a particular chamber showed a clear preference for the drug-linked chamber when later allowed to roam freely between it and a second chamber. Rats that were pretreated with Gly-Gln prior to receiving nicotine or morphine divided their time roughly equally between drug-linked and saline-linked chambers.
Gly-Gln Blocks the Rewarding Effects of Nicotine and Morphine: Rats trained to associate nicotine or morphine infusions with a particular chamber showed a clear preference for the drug-linked chamber when later allowed to roam freely between it and a second chamber. Rats that were pretreated with Gly-Gln prior to receiving nicotine or morphine divided their time roughly equally between drug-linked and saline-linked chambers.The researchers utilized an animal model called conditioned place preference (CPP; for more on CPP, see "Animal Experiments in Addiction Science (Archives)"). When pretreated with Gly-Gln, rats stopped preferring a cage in which they received morphine infusions over another in which they received a physiologically inert substance. The investigators concluded that Gly-Gln completely blocked the brainrewarding effects of morphine. They used the same experimental technique to demonstrate that Gly-Gln pretreatment also blocks the rewarding effects of nicotine in rats.
Some chemicals that block the rewarding effects of drugs of abuse also take away subjects' pleasure in normal healthy activities. Again using CPP, Dr. Millington's team demonstrated that Gly-Gln does not have this drawback, at least in regard to one food—a sweetened cereal—that rats enjoy.
Subsequently, Dr. Millington and his coinvestigators examined Gly-Gln's effect on withdrawal from morphine. They induced morphine dependence in the rats, injected some with Gly-Gln 72 hours later, and then administered the opioid antagonist naloxone to induce withdrawal. Across all measures, the Gly-Gln pretreated rats exhibited significantly fewer behavioral and physiological signs of withdrawal than the others. The researchers observed a similar effect when they induced withdrawal from nicotine with the agent mecamylamine.
Safe Analgesia, Too?
In separate studies, Dr. Millington and colleagues found evidence that Gly-Gln has potential for improving pain treatment by slowing the development of morphine tolerance. The investigators treated rats with morphine twice daily for 7 days and, each day, measured the rats' reaction to pain with tailflick latency tests. They observed that the pain-relieving effects of morphine declined 20 percent by the second day, an indication that tolerance had developed rapidly. However, rats pretreated with Gly-Gln did not begin showing evidence of morphine tolerance until the fourth day of treatment. Their level of pain relief had dropped to 75 percent of the maximum by day 4, compared with 39 percent for rats that were not pretreated.
These findings offer new hope for making pain treatment more effective, but without adding to the problems of painkiller abuse and prescription drug diversion. Dr. David Thomas of NIDA's Division of Basic Neuroscience and Behavioral Research explains, "It is like a tug-of-war; we want better pain treatment, but we do not want more addiction. If we can find a medication that improves the management of chronic pain without causing addiction or the negative physical side effects, then it really is a win-win situation. Gly-Gln may give us that."
"We need to learn much more about Gly- Gln's pharmacology before we can develop it into a drug that is useful clinically," says Dr. Thomas. "The profile of its effects brought out by Dr. Millington's work is striking. It makes a strong argument for taking the research to the next step: understanding its mechanism of action in the brain. In our world of preclinical animal research, we really could not ask for better findings. So far, everything we know about Gly-Gln is promising."
Sources
Cavun, S.; Goktalay, G.; and Millington, W.R. Glycyl-glutamine, an endogenous beta-endorphin-derived peptide, inhibits morphine-induced conditioned place preference, tolerance, dependence, and withdrawal. Journal of Pharmacology and Experimental Therapeutics 315(2):949- 958, 2005.
Goktalay, G., et al. Glycyl-glutamine inhibits nicotine conditioned place preference and withdrawal. European Journal of Pharmacology 530(1-2):95-102, 2005. [Abstract]
