Cocaine produces the long-term brain changes that underlie addiction in part by activating certain genes. Dr. Eric Nestler and colleagues at the University of Texas Southwestern Medical Center and Harvard Medical School have shown that the drug achieves this activation at least in part through a process called chromatin remodeling.
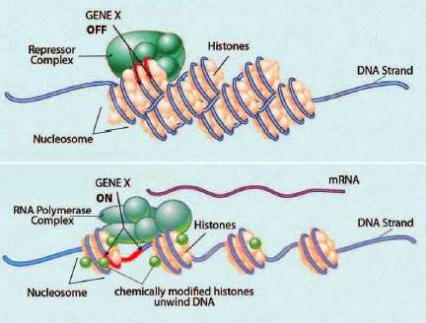 Chromatin and DNA Helix Form Chromosomes: Nucleosomes slide apart during chromatin remodeling, increasing transcription factors' access to a gene and thereby activating it. The DNA will then make mRNA, the blueprint for protein production.
Chromatin and DNA Helix Form Chromosomes: Nucleosomes slide apart during chromatin remodeling, increasing transcription factors' access to a gene and thereby activating it. The DNA will then make mRNA, the blueprint for protein production.The finding opens up a new avenue for potential therapies for addiction. "Our research suggests that testing chemical compounds that reverse chromatin remodeling is a promising approach to seeking treatments for drug abuse. This is already a major strategy for cancer therapy development," says Dr. Nestler.
Genes Regulate Crucial Proteins
Cocaine activates the genes that provide the templates for building the proteins cFos, ΔFosB, BDNF, and Cdk5, among others. Researchers have linked the resulting higher brain levels of some of these proteins with long-term consequences of chronic drug abuse. For example, accumulation of long-lasting ΔFosB correlates with cocaine craving and drug self-administration in animals, and may contribute to longlasting structural changes in cocaine abusers' brain reward systems. As researchers continue to trace out the consequences of cocaine-induced gene activation, Dr. Nestler and colleagues pursued a related inquiry: How does it happen? Their candidate explanation was chromatin remodeling, a basic mechanism cells use to alter levels of the body's vast array of proteins to suit new circumstances and challenges (see insert below).
Chromatin consists of the deoxyribonucleic acid (DNA) double helix that carries an organism's genes wrapped around complexes of histone proteins. The unit of chromatin is called the nucleosome, and chemical processes control how tightly packed nucleosomes are. Chromatin remodeling occurs when this packing becomes more or less compact. As the nucleosomes bunch up or spread out, some genes move into positions that increase—and others into positions that decrease—their ability to interact with RNA polymerase, the enzyme that executes the first step in protein building. This helps determine how much of the protein blueprinted by each gene will be made.
To test their hypothesis that cocaine activates genes by inducing chromatin remodeling, Dr. Nestler and his team compared tissue taken from the striatum of rats exposed to the drug and others given saline. Specifically, they assayed the tissue for the end products of two chemical reactions known to modify chromatin's shape: acetylation and phosphoacetylation of its primary molecular components, histone 3 (H3) and histone 4 (H4). Both of these reactions remodel chromatin in ways that increase gene expression.
The findings bore out the hypothesis. Within 30 minutes of a single injection, the chromatin associated with the cFos gene in the cocaine-exposed animals contained twice as much acetylated H4 than that in the control animals, and phosphoacetylated H3 also was higher. The time course of these effects jibed with previous observations that cocaine induces a rapid, transient increase in levels of the cFos protein. They were no longer present in tissues taken 3 hours after the injection, and they stopped occurring when animals were given repeated cocaine doses over an extended period. These data support scientists' conception of the cFos gene as an early responder to acute neural disruptions, with little or no direct role in situations of recurrent disruption.
As with cFos, a single cocaine injection elevated acetylated H4 in chromatin linked to the FosB gene, but the levels returned to baseline within 3 hours. Repeated cocaine did not induce H4 acetylation in FosB geneassociated chromatin, but did cause H3 acetylation. The researchers say that the switch from H4 acetylation after a single cocaine exposure to H3 acetylation after chronic exposure may mark a turning point in developing addiction.
Experience Restructures Chromatin
Chromosomes (pictured) are very long, continuous pieces of DNA that contain genes that help determine an individual's identity. Humans have 23 chromosome pairs with an estimated 30,000 genes.The DNA sequence wraps around proteins that give the chromosome a structure; together, they form chromatin. Cocaine and other external agents and experiences can alter the configurations of these proteins. Depending on the type of chemical change, the chromatin either bunches up or stretches out, activating or silencing genes along the DNA sequence.
Chromatin reshaping seems to underlie healthy adaptations such as learning and memory as well as disease processes—including cancer, seizures, schizophrenia, and depression. In another study, for example, Dr. Nestler's team found that social stress turned on a particular gene in the brains of mice through chromatin remodeling, a long-lasting change that corresponded with a behavioral indicator of depression. Antidepressant medication reversed both the behavioral sign of depression and the elevated gene activity, underscoring a key point about the modifications: experience and chemical agents can alter gene expression through chromatin remodeling, but such changes are reversible.
"More research is needed to identify the specific molecular basis of this switch," says Dr. Nestler. "However, prior work in my laboratory and with collaborators is starting to fill in a picture of why H3 acetylation and FosB's activation and subsequent triggering of ΔFosB after chronic cocaine might be important. We believe that this series of molecular events, and probably others, mediate the long-term behavioral and neural changes that underlie the transition from drug abuse to addiction," says Dr. Nestler. The experiments and assays also showed:
- A single cocaine injection did not affect BDNF or Cdk5 gene-associated chromatin, but chronic exposure induced H3 acetylation of both. Once initiated, the effects were long-lasting. The quantities of modified H3 in BDNF gene-associated chromatin in exposed animals increased from 3-fold of those of salinetreated animals at day 1 to 14-fold at day 7. Acetylated H3 related to the Cdk5 gene were more than two-fold those of saline 1 day after the last injection, and started to return to control levels only 7 days after cocaine cessation. Such persistent and robust gene activation long after the last dose of cocaine is striking in contrast with the relatively short-lived activation observed for cFos and FosB.
- Elevations in the ΔFosB protein selectively activated Cdk5—the only gene examined in the study that was turned on in this way. This finding suggests that ΔFosB may influence histone modifications by recruiting the chemical agents of chromatin remodeling to some target genes.
"Understanding how cocaine turns on these genes could help addiction researchers develop potential treatments that counteract the effects of drug abuse at the molecular level. Agents that reverse chromatin remodeling are available, and we are examining whether they block cocaine's cellular effects," says Dr. Nestler.
"Taken together with other studies showing that drugs induce long-term structural changes to brain cells, Dr. Nestler's findings show that chromatin remodeling is one way that such neural modification might occur. Such alterations are not necessarily permanent, and studies are needed to determine whether abstinence or other behavioral modifications further restructure chromatin to a state similar to that seen prior to drug exposure," says Dr. Joni Rutter of NIDA's Division of Basic Neuroscience and Behavioral Research. Whether nonstimulant drugs of abuse also act through chromatin remodeling is another important area for future research, she says.
A Connection With Cocaine-Related Behavior
In other experiments, Dr. Nestler and colleagues linked chromatin remodeling to cocaine's behavioral effects by examining its role in a laboratory stand-in for human cueinduced drug seeking called conditioned place preference (CPP). By exhibiting CPP—lingering in a part of a cage where it has received a drug—an animal indicates that it is seeking more of the drug (see "Animal Experiments in Addiction Science (Archives)"). The researchers hypothesized that augmenting or preventing histone modifications during drug administration sessions would increase or decrease CPP, respectively.
Cocaine Turns on Genes by Altering Chromosomal Proteins:
The researchers found chemical modifications to histone 3 (H3) and histone 4 (H4)—major proteins that form the structure of chromosomes—at areas linked with four genes. Acetylation of H3 and H4 and phosphoacetylation of H3 alter the proteins' chemical structure, facilitating gene activation.
| Gene | Results | |||||
|---|---|---|---|---|---|---|
| 30 min. | 1 hr. | 90 min. | 3 hr. | 1 day | 7 days | |
| cFos | Acetylated H4 Phosphoacetylated H3 |
Normal Histones |
Normal Histones |
|||
| FosB | Acetylated H4 | |||||
| BDNF | No change | |||||
| Cdk5 | No change | |||||
| Gene | Results | |||||
|---|---|---|---|---|---|---|
| 30 min. | 1 hr. | 90 min. | 3 hr. | 1 day | 7 days | |
| cFos | No change | |||||
| FosB | Acetylated H3 | Normal Histones | ||||
| BDNF | Acetylated H3 | Normal Histones |
||||
| Cdk5 | Acetylated H3 | Normal Histones |
||||
| Gene | Results | |||||
|---|---|---|---|---|---|---|
| 30 min. | 1 hr. | 90 min. | 3 hr. | 1 day | 7 days | |
| cFos | Effects too transient to examine | |||||
| FosB | Effects too transient to examine | |||||
| BDNF | Acetylated H3: Levels rise to 3-fold those associated with saline injection after 1 day, and to 14-fold after 7 days. | |||||
| Cdk5 | Acetylated H3: Levels rise to 3-fold those associated with saline injection after 1 day, and return to normal after 7 days. | Normal Histones | ||||
The investigators administered cocaine to mice daily for 4 days. Before each administration, they treated one group with a drug that enhances histone acetylation (trichostatin A, TSA) and another with a virus that expresses an enzyme that blocks this particular modification (herpes simplex virus, HSV). When placed back in the test cage on day 5, the group given TSA doubled the time spent in the drug-associated cage, on average, relative to the control group. In contrast, the group given the HSV vector lingered in the test cage for one-third of the time spent by its control group.
The findings suggest a causal link between histone acetylation in the striatum and sensitivity to cocaine's behavioral effects.
"The team had already demonstrated that chromatin remodeling plays a role in the rewarding aspects of cocaine abuse by including a group of animals that selfadministered the drug in the study. Their CPP experiment further strengthens the connection between histone restructuring and behavioral aspects of addiction and suggests that agents that reverse chromatin restructuring hold promise as potential therapies," says Dr. Rutter.
Source
Kumar, A., et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48(2):303-314, 2005. [Abstract]