Morphine and other opioids suppress the immune system, the body's innate defense against infections. Because of this effect, doctors weigh the pain-relief benefits of opioids against the added risk of infection they pose to patients, particularly those being treated for severe burns or certain cancers. Opioid abusers, many of whom are already infection-prone due to unclean needles, repeated injections, and poor nutrition and living conditions, are rendered even more vulnerable by these drugs.
Morphine affects the body's immune cells in many ways, both direct and indirect. Recently, NIDA-funded scientists pinpointed the biochemical trigger that sets off a chain reaction that ultimately inhibits an immune cell that is key in fighting viruses and cancer. If validated in future studies, the work could lead to interventions to bolster the immunity of those who regularly take opioids.
Morphine, Dopamine, and Natural Killers
Morphine suppresses the activity of three different types of white blood cells: T lymphocytes, B lymphocytes, and natural killer (NK) cells (see box). NIDA-funded investigators Dr. Donald Lysle, Dr. Timothy Saurer, and their colleagues at the University of North Carolina (UNC), Chapel Hill, concentrated on NK cells, and found that:
- morphine-induced immunosuppression follows activation of dopamine-1 (D1) receptors in the shell of the nucleus accumbens (NAc); and
- a train of biochemical events links stimulation of these D1 receptors with reduced NK cell activity in the spleen.
In previous studies, the UNC team had established that blocking one of morphine's pharmacological effects in the brain—a surge of dopamine into the NAc—averts suppression of NK cells in the spleen. This being the case, the researchers reasoned that morphine-induced NK suppression must start with something that dopamine does in the NAc.
Natural Killer Cells Eliminate Compromised Cells
Natural killer (NK) cells constitute a rapid-response force against cancer and viral infections. These specialized white blood cells originate in the bone marrow, circulate in the blood, and concentrate in the spleen and other lymphoid tissues. NK cells key their activities on a subset of the histocompatibility proteins that occur on the surfaces of healthy cells but that virus- and cancer-weakened cells shed. When NK cells encounter cells that lack histocompatibility proteins, they attack and destroy them—thus preventing the cells from further spreading the virus or cancer. NK cells are distinguished from other immune system cells by the promptness and breadth of their protective response. Other white blood cells come into play more slowly and target specific pathogens—cancers, viruses, or bacteria—rather than damaged cells in general.
Dopamine's main action in the NAc is to interact with proteins called dopamine receptors, which come in several subsets—D1, D2, for example. Accordingly, Dr. Lysle and colleagues conducted experiments to determine whether any of these receptors were involved in morphine inhibition of NK cells. They began by giving rats morphine (15 mg/kg) or saline. One hour later, they measured NK cell activity in the animals' spleens and found it to be lower by about 40 to 50 percent, on average, among the morphine-exposed animals, compared with those given saline. They then conducted a series of trials that showed:
- Morphine-induced NK cell suppression depends on the activation of D1 receptors. Rats injected with a compound (SCH-23390) that prevents dopamine from accessing D1 receptors did not develop immunosuppression when subsequently injected with morphine. In contrast, rats pretreated with a compound (raclopride) that blocks D2 receptors did lose NK cell activity.
- Specifically, D1 receptors that reside in the part of the NAc called its shell must be activated for morphine NK cell suppression to occur. Morphine inhibition of NK cell activity was abolished when researchers infused SCH-23390 into rats' NAc shell (see graph, left panel), but not the NAc core.
- D1 receptor activation in the shell of the NAc lowers NK cell activity even in the absence of morphine. Rats given no morphine but a test compound (SKF 38393) that activates D1 receptors demonstrated 35 to 39 percent less NK cell activity than a comparison group of rats injected with saline.
Pathway Through the Body
Dr. Lysle and colleagues next turned their attention to the signaling chain that links D1 activity in the NAc to NK cell inhibition in other organs. Prior studies had indicated that morphine activates the sympathetic nervous system, and sympathetic nerves communicate signals from the brain to NK and other white blood cells in the spleen. The specific chemical involved in conveying the message of NK inhibition from brain along the sympathetic system to the spleen, however, was unknown.
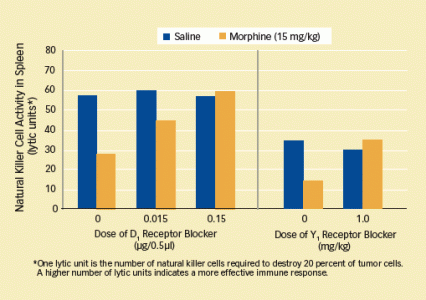 How to Prevent Morphine-Induced Suppression of Natural Killer Cells: Blocking the D1 receptor in a particular area of rats' brains (the nucleus accumbens shell) prevented morphine-induced inhibition of natural killer cells—key immune system cells that fight viral infections and cancers. Preventing neuropeptide Y from interacting with its Y1 receptors in the body also counters morphine-induced inhibition of natural killer cells.
How to Prevent Morphine-Induced Suppression of Natural Killer Cells: Blocking the D1 receptor in a particular area of rats' brains (the nucleus accumbens shell) prevented morphine-induced inhibition of natural killer cells—key immune system cells that fight viral infections and cancers. Preventing neuropeptide Y from interacting with its Y1 receptors in the body also counters morphine-induced inhibition of natural killer cells.Dr. Lysle and colleagues focused on neuropeptide Y (NPY) as a likely candidate because it is released in the brain when sympathetic nerves are activated, is found at the nerves of the spleen, and interacts with receptors (Y1) in the membranes of white blood cells. "Most important in elevating NPY as a candidate, however, was other researchers' finding that it suppressed the ability of natural killer cells to attack cultured tumor cells," says Dr. Saurer. "This suggested that it might modulate natural killer activity and perhaps the suppressive effects of morphine in rats."
The investigators confirmed their hypothesis with an experimental strategy parallel to the one they had used to link the brain's D1 receptors to morphine-induced NK cell immunosuppression: They showed that neither NK cell inhibition induced by morphine nor D1-related NK inhibition occurs when NPY is prevented from interacting with its main receptor (Y1) (see graph, right panel).
The researchers next wondered whether Y1 receptor activation might underlie suppression of other white blood cells as well as suppression of NK cells. To find out, they stimulated rats' Y1 receptors with injections of NPY (20 or 200 mg).
The infusion inhibited NK cell activity by 55 percent, on average, but had no effect on T and B white blood cells in the spleen. Similarly, rats infused with morphine while their Y1 receptors were blocked did not develop NK cell inhibition, but their T and B white blood cells became inhibited.
The specificity of NPY's actions—affecting NK cells but not other white blood cells—is not surprising, says Dr. Lysle, because the brain generally seems to communicate with different immune system components in the body in very selective ways. "Our team has identified the language for the suppression of natural killer cells, but other drugs of abuse and different immune system responses may speak distinct languages—ones that may be discovered in future research," he says.
"The team's findings suggest a brain-to-body pathway whereby morphine weakens the body's defenses," says Dr. Paul Schnur of NIDA's Division of Basic Neuroscience and Behavioral Research. "In future studies, researchers might examine immune responses after chronic self-administration of morphine to model more closely the possible immunosuppressant effects of drug abuse."
Sources
Saurer, T.B., et al. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. Journal of Neuroimmunology 173(1- 2):3-11, 2006. [Abstract]
Saurer, T.B.; Ijames, S.G.; and Lysle, D.T. Neuropeptide Y Y(1) receptors mediate morphine-induced reductions of natural killer cell activity. Journal of Neuroimmunology 177(1-2):18-26, 2006. [Abstract]
Conditioned Responses Can Also Impair Natural Killer Cells
An environment linked with an immune system trigger such as an allergen will, after repeated pairings, incite an immune response in the absence of the trigger. Similarly, an environment associated with an immunosuppressant such as morphine weakens immune responses.
In these conditioned responses, the brain generates the initiating signal for immunosuppression. But where in the brain does the message start? And how does it travel to the distant organs where immune cells reside?
Dr. Donald Lysle, Dr. Timothy Saurer, and their colleagues at the University of North Carolina, Chapel Hill, proposed that the biochemical chain of events in conditioned immunosuppression recapitulates the one set off by the original immune-system-weakening stimulus. To put this idea to the test, they conducted experiments on rats that were similar to those they had performed to establish the mechanisms of morphine-induced natural killer (NK) cell suppression.
The researchers gave rats a dose of morphine (15 mg/kg) to suppress NK cell activity. They immediately placed the rats for 1 hour in special conditioning chambers that were distinct from the home cages—with different walls and floors and featuring a unique sound and smell. They returned the animals to the home cages and repeated the process 2 days later.
After two conditioning sessions and a 12-day break, the researchers left some rats in the home cages and returned others to the morphine-paired conditioning chambers. None of the rats was given morphine at this stage. After 1 hour, NK cell activity in the spleen was lower by about 50 percent, on average, among the animals in the morphine-linked chambers, compared with those in the home cages. The researchers then conducted a series of trials that showed:
- For conditioned NK cell suppression to occur, D1 receptors must be activated.
- In conditioned NK cell suppression, D1 receptors that reside in the part of the nucleus accumbens called its shell are critical.
- In conditioned NK cell suppression, Y1 receptors in the spleen must be activated.
The findings confirm the researchers' hypothesis that the same biological train of events that underlies morphine immunosuppression also drives conditioned immunosuppression, which occurred, in this case, in response to an environment previously paired with morphine exposure. "The findings expand on the team's prior descriptions of the neurobiological mechanisms underlying the ability of opiates and environments associated with them to suppress various immune responses in animals," says Dr. Paul Schnur of NIDA's Division of Basic Neuroscience and Behavioral Research. "They suggest that opiate abusers are vulnerable to a weakened immune system in places previously associated with taking the drug."
Source
Saurer, T.B. et al. Neuroimmune mechanisms of opioid-mediated conditioned immunomodulation. Brain, Behavior, and Immunity 22(1):89-97, 2008. [Abstract]