For millennia, opioid medications have provided the most potent tools for pain relief. Yet, although they are the best we have, opioids fall short of being ideal analgesics: They have unpleasant side effects, their efficacy diminishes with ongoing use, they cause physical dependence, and they pose significant risks for abuse and addiction. Moreover, opioids do not quell all types of pain. For example, they provide only partial, often temporary relief to people with neuropathic pain, which commonly develops when disease or trauma injures nerves and typically produces burning, stinging, or tingling that doesn't cease.
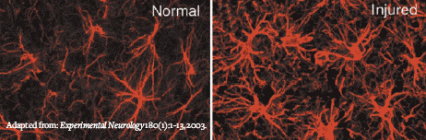 After Spinal Cord Damage, Glial Cells Called Astrocytes Proliferate and Expand
After Spinal Cord Damage, Glial Cells Called Astrocytes Proliferate and ExpandNIDA-supported research teams are advancing along separate paths to develop new compounds that match or exceed the pain relief provided by opioids while avoiding their shortcomings. Dr. Linda R. Watkins and colleagues at the University of Colorado at Boulder are pursuing the implications of their discovery that glia—nonneuronal nervous system cells—play a previously unrecognized key role in the generation of neuropathic pain and in opioid tolerance and withdrawal. Dr. Philip S. Portoghese and collaborators at the University of Minnesota and Louisiana State University are tinkering with opioid molecules to capitalize on recent findings showing that opioids produce pain relief by mechanisms separate from those that produce their less desirable effects. During the past year, both groups have reported highly promising results.
The Glial Strategy
Dr. Watkins' research team, in collaboration with Dr. Kirk W. Johnson and colleagues at Avigen Inc. of Alameda, California, recently relieved neuropathic pain in rats by giving them AV411 (also called ibudilast; see first box below), a compound that inhibits glial cell activity. In other animal studies by Dr. Watkins and other researchers, various compounds that inhibit glial cells have reduced or abolished pain that simulated many types of human chronic neuropathic pain. They also have boosted eight-fold the acute pain relief afforded by morphine.
Dr. Watkins and colleagues discovered the analgesic potential of glial cell inhibition through pioneering research that implicates glial cells in the amplification and long-term maintenance of pain. Their investigations showed that tissue or nerve inflammation or damage that is associated with neuropathic pain heightens activity of two types of glial cells—microglia and astrocytes—in the spinal cord. The activated glia release biochemicals—including proinflammatory cytokines and chemokines, nitrous oxide, and prostaglandins—that excite spinal cord neurons to transmit strong and persistent pain signals to the brain. The finding that glial cells modulate pain in the spinal cord prompted the researchers to explore glial cell inhibition as a broad strategy for controlling many types of pain.
Dr. Watkins' findings extend the roster of important functions attributed to glial cells. It was previously known, for example, that glia maintain hospitable microenvironments for neurons and provide them with molecular support for carrying out their cellular functions. Another glial cell activity, clearing cellular debris away from neurons, can last for years—a fact, the researchers suggest, that may have important implications for the long duration of chronic pain.
Enhancing Opioids and Making Them Safer
Working with researchers at the University of Adelaide in Australia, Avigen has completed an exploratory clinical trial to test AV411 in patients with neuropathic pain. Patients in the study, who were predominantly diagnosed with painful diabetic neuropathy, received fixed regimens of AV411 as well as concomitant analgesics, including opioids. Preliminary results indicated that patients receiving AV411 reduced their opioid use, suggesting that AV411 relieved pain or reduced opioid tolerance.
The idea that glial modulatory compounds might counteract opioid tolerance emerged from studies to understand the biochemical basis for that phenomenon. In experiments with animals, Dr. Watkins' team demonstrated that opioids, like neuropathy, stimulate glial cells to release substances that in turn incite spinal cord neurons to amplify pain signaling. This response combines with the neuroexcitatory glial response triggered by neuropathy itself to offset the opioid's analgesic efficacy.
AV411 for Pain Relief Without Opioid Side Effects
AV411 (also called ibudilast) is prescribed in Asia to treat asthma and post-stroke dizziness. It is also being tested in Eastern Europe for treatment of multiple sclerosis and in the United States for other neurological conditions. The medication inhibits glial cells from triggering inflammatory responses that target neurons.
In Dr. Watkins' studies with laboratory animals, AV411 alleviated some types of chronic pain more effectively than morphine. Given in combination with morphine, AV411 enhanced analgesia in acute pain, retarded the development of morphine tolerance, reduced the severity of morphine withdrawal, and made morphine less rewarding.
Alleviation of chronic neuropathic pain
Two types of chronic pain from traumatized nerves
To simulate traumatic nerve damage that causes two types of chronic pain in people, the researchers operated on two groups of rats. They surgically tied off either the sciatic nerve or two nerves that emerge from the spinal column.They then measured how hard they might press fibers against the paws served by these nerves before the animals felt uncomfortable enough to pull them away.
Among the findings were:
- In both groups of rats, twice-daily AV411 injections enhanced the animals' pressure tolerance up to three-fold for up to 16 hours, depending on the dosage;
- In rats with sciatic nerve damage, oral AV411 increased the pressure tolerance for 2 to 4 hours and was well-tolerated with only a few, passing adverse effects.
Chronic pain from cancer chemotherapy
The researchers gave rats an anticancer agent, paclitaxel, which irritates peripheral nerves. The animals' pressure tolerance declined steadily with continuing chemotherapy but stabilized and reverted toward prechemotherapy levels following initiation of AV411 12 or 19 days into the treatment.
Improvement of the efficacy and safety of morphine
Enhancement of morphine analgesia for acute pain
The researchers measured how much heat a rat would tolerate on its tail before moving the tail away. Animals given AV411 plus morphine tolerated heat for a longer period of time than rats given morphine alone. AV411 boosted morphine's pain-killing potency about eight-fold.
Suppression of morphine tolerance
The researchers induced damage to rats' sciatic nerve. They then treated some of the animals with repeated injections of AV411 plus morphine and others with morphine alone. For the first 12 days, animals receiving both regimens withstood as much pressure on the paws served by the disrupted nerves as they had prior to surgery, indicating ample pain suppression. By day 16, rats that received morphine alone flinched away from lower pressures, demonstrating that the relief given by the medication was waning with repeated exposures; animals on the combination treatment, however, continued to withstand as much pressure as they had on day 12.
Alleviation of morphine withdrawal
The researchers gave healthy rats either morphine alone or morphine with AV411 for 5 days. Next, they stopped the injections and observed the animals for signs of withdrawal—including excessive chewing, jumping, grooming, and exploring. The animals that received only morphine exhibited such signs much more markedly than did the animals that received morphine with AV411.
Treatment of addiction?
Using a standard behavioral assay, the researchers found that morphine was less rewarding to rats who received it together with AV411. Similarly, AV411 dampens the neurochemical surge that underlies morphine reward in rats. Taken together, the preliminary findings suggest that AV411 has promise as a treatment for morphine addiction.
AV411's ability to enhance opioid efficacy could be especially valuable for chronic pain patients with histories of drug abuse. At a NIDA-sponsored panel at the annual Society for Neuroscience meeting on November 2, 2007, Dr. Watkins reported preliminary results suggesting that the medication counters the opioid-induced reward sensations that can raise such patients' risk for relapse. Using an established experimental model to assess reward, the researchers repeatedly infused rats with morphine in a test chamber, then let the animals decide whether to spend time in that chamber or another.
Rats that had been pretreated with AV411 spent roughly equal amounts of time in both chambers; their lack of preference for the test chamber suggests that the morphine experience was not rewarding for them. In a related study at the University of Colorado-Boulder, spearheaded by Dr. Sondra T. Bland, AV411 suppressed—by about half—a biochemical signature of drug reward, the morphine-induced dopamine surge in rats' nucleus accumbens. This research is the first to demonstrate a relationship between glial cell activity and opioid reward.
The pharmacological action that underlies AV411's beneficial effects against pain, as well as its influence on opioid reward and tolerance, appears to be partial inhibition of a particular receptor on glial cells. This receptor, called TLR4, is a member of a family of receptors called toll-like receptors (TLRs). TLR4s sit on the surface of the glial cells and detect external dangers, such as bacteria; some also respond to indicators of tissue damage, such as bits of dead cells. Once activated, TLR4s trigger an immune response to combat the invader that caused the tissue damage. Dr. Mark R. Hutchinson's research in Dr. Watkins' laboratory suggests that TLRs are also the sites where opioids and nerve damage converge to trigger glial cell responses that amplify and maintain long-lasting pain, as well as a likely site where opioids prompt the responses by glia that enhance opioid reward and tolerance.
Beyond the Central Nervous System: Analgesic Opportunities on the Periphery
The discovery of cannabinoid and vanilloid nerve cell receptors during the 1990s opened up still another avenue for the development of nonaddictive pain medication. Animal research suggests that these receptors affect nerve cells' responses to pain and that modifying the activity of these receptors can produce analgesia.
Focus on one type of cannabinoid receptor
Efforts to modulate the cannabinoid (CB) system for analgesia are currently centered on the CB2 receptor, one of the system's two known receptors. Both CB1 and CB2 receptors respond to naturally occurring neurochemicals and to drugs (for example, D9-tetrahydrocannabinol, the active ingredient in marijuana). CB2 receptors are generally not found in the brain and spinal cord but are located on nerve cells throughout the body and seem to modulate pain through actions close to the site of injury or inflammation. Because of that localization, stimulation of the CB2 receptor is not expected to cause the sedation and impaired motor coordination effects associated with CB1 receptor stimulation (see "Novel Cannabinoid Appears Promising for Treatment of Chronic Pain (Archives)").
"A focus on CB2 receptors in pain therapeutics seems a promising strategy," says Dr. Rao Rapaka of NIDA's Division of Basic Neuroscience and Behavioral Research. "Because CB2 receptors are present on immune system cells, scientists were concerned that stimulating them for pain relief may induce immunological side effects. Recent animal studies, however, indicate that the compounds do not induce such side effects."
Chili peppers, Moroccan plant provide pain killers
Attempts to reduce pain by modulating vanilloid receptor activation are focused on a molecule called transient receptor potential vanilloid 1 (TRPV1). TRPV1s on nerve cells throughout the body detect heat and irritating touch; when stimulated, they signal those sensations to the brain. Capsaicin, the active component of chili peppers and some topical analgesic ointments, is a member of the vanilloid family of compounds that activates TRPV1s. While capsaicin produces a short-lived burning or tingling sensation upon first contact with tissues, it does not damage them; it appears to overstimulate nerve cells so that they do not again signal pain for several hours.
A potent, naturally occurring chemical called resiniferatoxin (RTX) also stimulates TRPV1s. A cactus-like plant (Euphorbia resinifera) in Morocco contains high concentrations of RTX, and researchers are using its resin to develop new analgesic medications. Like capsaicin, RTX-based treatments would likely have minimal potential for abuse and few side effects. Scientists expect that injectable RTX-based medications would provide more potent pain relief than capsaicin-based topical treatments. "A single RTX local injection may offer a long-term treatment for some types of chronic pain without many of the side effects that are associated with opioid analgesics," says Dr. David Thomas of NIDA's Division of Basic Neuroscience and Behavioral Research. "Animal studies have shown that RTX alleviates both inflammatory and neuropathic pain. If clinical trials show that RTX is safe and effective, patients with certain types of pain will have a much needed, powerful alternative to current pain treatments."
"Blocking TLR4 separates morphine's good and bad effects, and my colleagues and I believe that drugs that block TLR4 will prove to have great clinical utility both for making morphine better for pain control and as standalone drugs for treating neuropathic pain," says Dr. Watkins. In addition to working with AV411, Dr. Watkins and colleagues are collaborating with Dr. Kenner Rice of NIDA to create and test highly specific TLR4 antagonists that may maximize the potential of this pharmacological action.
"Our findings suggest that suppressing glial activation with AV411 will help relieve neuropathic pain, as well as other types of pain, and also reduce feelings of pleasure from opioids that drive craving and drug-seeking among abusers," says Dr. Watkins. "Our results, when taken together with the findings of other researchers, suggest that AV411 inhibits the cascade of molecular events surrounding nerve damage in the body as well as the inflammatory response in the brain. AV411 successfully reaches the brain, whereas other inflammation inhibitors do not, which likely contributes to its beneficial effects in animals."
Dr. David Thomas of NIDA's Division of Basic Neuroscience and Behavioral Research notes that the results of this animal research look promising. "But clinical studies are needed to determine whether AV411 will provide relief for people with neuropathy," he adds.
Building Better Opioids
To create improved opioids, Dr. Portoghese and collaborators at the University of Minnesota and Louisiana State University are using newly discovered details about the receptors through which opioids exert their effects on neurons. The team's recently designed compounds provided more pain relief than morphine, and their analgesic effect did not diminish after repeated administration in laboratory tests. Mice given the compounds also showed no behavioral signs of physical dependence.
The researchers' design strategy is to construct single compounds that target both components of a particular dual-opioid receptor. Specifically, the compounds stimulate the mu component and inhibit the delta component of the mu-delta dimer, or linked-pair, receptor.
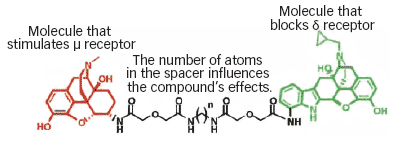 Dual-Component Compounds Open New Avenues for Potential Medications: New opioid compounds called mu-delta agonist-antagonists (MDANs) comprise two molecules tied together by a chain of atoms called a spacer (chemical structure shown). The compounds target the μ-δ opioid heterodimer, an adjoining pair of receptors, in the neuronal membrane.
Dual-Component Compounds Open New Avenues for Potential Medications: New opioid compounds called mu-delta agonist-antagonists (MDANs) comprise two molecules tied together by a chain of atoms called a spacer (chemical structure shown). The compounds target the μ-δ opioid heterodimer, an adjoining pair of receptors, in the neuronal membrane.Dual—or dimeric—receptors are a recent discovery. Neuroscientists long supposed that all receptors were singular entities. The finding that some receptors form pairs opened up new possibilities for medication development. Scientists observed that when a specially designed two-part compound simultaneously engages both receptors in a linked pair, the cell reacts differently than when either receptor unit is triggered singly.
In fundamental research conducted in the 1990s, Dr. Portoghese and colleagues set the stage for the development of a new class of opioids. The results of an animal study showed that simultaneous stimulation of the mu receptor by one compound and inhibition of the delta receptor by another produced less tolerance and dependence than mu receptor stimulation alone. With the subsequent discovery of linked-pair receptors, Dr. Portoghese and colleagues recognized that targeting dimers, rather than two independent receptors, could capitalize on the special response characteristics of a dimer and balance mu activation and delta inhibition.
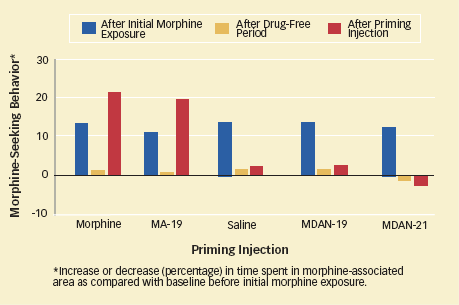 Animal Study Suggests MDANS Will Not Threaten Recovery: In a test of MDAN's potential to trigger renewed drug use following abstinence, mice underwent morphine exposure to establish drug seeking followed by a drug-free period. They then received a priming injection. As expected, those primed with morphine or another compound (MA-19) that binds solely to the mu opioid receptor returned to intense drug seeking. Those who received MDAN-19 or MDAN-21, which bind at both mu and adjoining delta opioid receptors, did not.
Animal Study Suggests MDANS Will Not Threaten Recovery: In a test of MDAN's potential to trigger renewed drug use following abstinence, mice underwent morphine exposure to establish drug seeking followed by a drug-free period. They then received a priming injection. As expected, those primed with morphine or another compound (MA-19) that binds solely to the mu opioid receptor returned to intense drug seeking. Those who received MDAN-19 or MDAN-21, which bind at both mu and adjoining delta opioid receptors, did not.Each molecule in the team's new class of opioids comprises a mu agonist molecule and a delta antagonist molecule linked by a chain of atoms called a spacer (see diagram, above). The compounds are called mu-delta agonist-antagonists, or MDANs. To maximize efficacy, the team sought spacer lengths such that once the first molecule settles into its docking area on the dual receptor, the second has just enough chain to reach its docking area. In the experiments, this criterion was met by a spacer length of 16 to 21 atoms, and the researchers designated the compounds, accordingly, MDAN-16 through MDAN-21. Taking these compounds into the laboratory, they observed that:
- Several MDANs blunted pain. Infusions of MDAN-16 through -21 directly into the brain increased the time that mice could withstand heat from a strong light beam on their tails. MDAN-21's analgesic potency was 50-fold that of morphine.
- After chronic exposure to MDAN-19, -20, or -21, the mice developed only minimal signs of physical dependence. The researchers delivered infusions of either saline, morphine, or an MDAN into the brains of mice for 3 days and the next day pharmacologically precipitated withdrawal. The mice on MDANs exhibited fewer withdrawal symptoms ( jumps) than those on morphine. Generally, mice given MDAN-19, -20, or -21 made the fewest jumps.
- Chronic administration of MDAN-19, -20, or -21 did not induce tolerance to the compound's pain-killing effects. Animals maintained on these MDANs via infusions for 3 days demonstrated pain relief from a new dose delivered 4 hours after the final infusion. In contrast, morphine's pain-killing effects dropped six-fold in the same experimental protocol.
- MDAN-19 and MDAN-21 show minimal potential for abuse and have little potential to induce a return to drug-seeking. In a standard behavior test, mice increased their drug-seeking after receiving morphine but not after receiving MDAN-19 or MDAN-21. Other experiments (see graph) suggest that the new compounds are unlikely to induce relapse among people recovering from opioid addiction.
"Dual-component compounds and dimers open up a new and exciting universe in medication development," says Dr. Portoghese. "My colleagues and I believe that there may be other combinations of opioid receptors that when stimulated with such compounds might alleviate pain without tolerance and dependence."
"Dr. Portoghese's results suggest that MDAN compounds could form a single drug with two functions—alleviation of pain and reduction of opiate withdrawal, tolerance, and side effects," says Dr. Paul Hillery of NIDA's Division of Basic Neuroscience and Behavioral Research. "The MDAN compounds are also useful tools for investigators who study how stimulating the receptor complexes activates or inhibits neurons."
Sources
Hutchinson, M.R., et al. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. The Scientific World Journal 7:98-111, 2007. [Abstract]
Ledeboer, A., et al. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biology 2:279-291, 2007. [Full Text (PDF, 270KB)]
Ledeboer, A., et al. Ibudilast (AV-411): A new class therapeutic candidate for neuropathic pain and opioid withdrawal symptoms. Expert Opinion on Investigative Drugs 16(7):1-16, 2007. [Abstract]
Lenard, N.R., et al. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. European Journal of Pharmacology 566(1-3):75-82, 2007. [Abstract]
Daniels, D.J., et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proceedings of the National Academy of Sciences 102(52):19208-19213, 2005. [Full Text (PDF, 319KB)]
