NIDA-supported scientists have demonstrated that the immune system participates in the shaping of brain circuits during a child's development. The finding represents a breakthrough in understanding how our early experience conditions our responses to events later in life, and it may also shed light on the origins of neurodegenerative diseases, such as Alzheimer's.
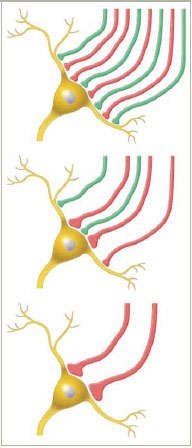 Winnowing the Connections: In a newborn mouse, neurons in certain areas of the lateral geniculate nucleus receive synapses from nerve cells originating in either eye (red and green). The mouse's eyes open when it's about 12 days old (top), and by day 17 (middle), some synapses from one eye have become stronger and begin to predominate. After neural pruning (bottom), only the strongest synapses remain, and most LGN neurons in these areas receive input from only one eye.
Winnowing the Connections: In a newborn mouse, neurons in certain areas of the lateral geniculate nucleus receive synapses from nerve cells originating in either eye (red and green). The mouse's eyes open when it's about 12 days old (top), and by day 17 (middle), some synapses from one eye have become stronger and begin to predominate. After neural pruning (bottom), only the strongest synapses remain, and most LGN neurons in these areas receive input from only one eye.Dr. Ben A. Barres and postdoctoral fellow Dr. Beth Stevens at Stanford University studied the phenomenon called neural pruning, which eliminates unused and little-used synaptic connections during development. To their surprise, the investigators found that the mechanism responsible for neural pruning is the same one that marks and destroys damaged and infected cells in the body throughout life. Their findings also indicate that the mechanism, called the immune complement cascade, may destroy healthy brain circuits in adulthood, with drastic consequences.
Surprising Sculptor
We are all born with brain circuits ready to respond to a vast variety of experiences. As we call upon some circuits and not others, the former strengthen and the latter languish. The presence of unused or little-used circuits can interfere with brain efficiency, so the brain trims them away.
One example of neural pruning is retinogeniculate segregation, a process that remodels visual circuits during development. Visual information from the retinas travels along nerve fibers to brain areas called the right and left lateral geniculate nuclei (LGN). In the period immediately after birth, neurons in some areas of the LGN possess structures—synapses—to receive information from both eyes. As the infant develops, however, the brain eliminates many of these synapses. Most neurons lose the synapses that receive information from one eye, and from then on receive information from the other eye only.
Drs. Stevens and Barres focused on retinogeniculate segregation and asked the question: What mechanism underlies this process? The electrical activity associated with normal vision certainly plays a role, but molecules must also be involved. Which ones?
To begin their inquiry, the researchers hypothesized that cells called astrocytes start the segregation process by activating genes for molecules that eliminate underused synapses. Assuming this hypothesis, they exposed purified mouse retinal ganglion cells to astrocytes. To their surprise, they found that astrocytes activated the genes for all three subunits of the protein, called C1q, that initiates the immune complement cascade.
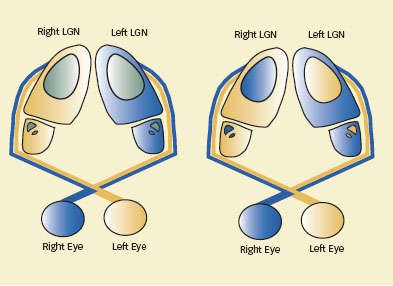 Retinogeniculate Segregation: In a newborn mouse (left), most of the neurons in the lateral geniculate nucleus (LGN) receive input from only one eye (tan or blue), but cells in some locations (green) receive input from both eyes. After retinal pruning (right), neurons in those areas receive input from one eye only.
Retinogeniculate Segregation: In a newborn mouse (left), most of the neurons in the lateral geniculate nucleus (LGN) receive input from only one eye (tan or blue), but cells in some locations (green) receive input from both eyes. After retinal pruning (right), neurons in those areas receive input from one eye only.Additional experiments produced further evidence that C1q is instrumental in retinogeniculate segregation. They showed high C1q levels in the retinogeniculate system, and the rest of the nervous system, precisely during the age span when the mouse brain accomplishes neural pruning. The levels—along with those of other complement cascade proteins—then declined, virtually disappearing by adulthood.
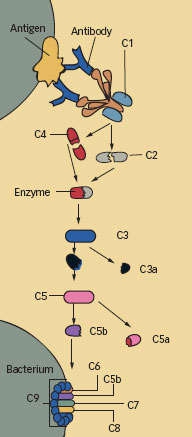 The Complement System at a Glance: The proteins newly identified as instigators of neural pruning in the brain are integral to the cascade of activity that the immune system uses to defend the body. The first complement protein, C1, is a complex of C1q and two other proteins. C1 initiates enzymatic activity that acts on C2 and C4 to create a new enzyme that then acts on C3. The products of that reaction, in turn, mark a pathogen's surface for immune attack, stimulate inflammation, or join with other complement components to break down C5. To kill an invading bacterium, the system finally produces a cylinder that punctures the pathogen's cell membrane.
The Complement System at a Glance: The proteins newly identified as instigators of neural pruning in the brain are integral to the cascade of activity that the immune system uses to defend the body. The first complement protein, C1, is a complex of C1q and two other proteins. C1 initiates enzymatic activity that acts on C2 and C4 to create a new enzyme that then acts on C3. The products of that reaction, in turn, mark a pathogen's surface for immune attack, stimulate inflammation, or join with other complement components to break down C5. To kill an invading bacterium, the system finally produces a cylinder that punctures the pathogen's cell membrane.These results suggested a mechanism for neural pruning: Astrocytes trigger the complement cascade, which eliminates underused synapses in the same way it disposes of dying or mutated cells and viral or bacterial invaders. To confirm this, the researchers bred mice that lacked the gene that produces C1q. As predicted by the theory, these mice did not accomplish neural pruning. Similar experiments demonstrated that C3, another component of the complement cascade, is also required for neural pruning. The team concluded that the classic complement cascade mediates synapse elimination in the developing brain.
"Neural pruning is a good thing when the brain is developing. Synaptic connections that we use frequently are strengthened, and those we don't use enough to justify maintaining are simply eliminated," says Dr. Stevens. However, further research by the Stanford team and others indicates that the same synaptic pruning mechanism that is beneficial in youth may recur at older ages and, when it does, may give rise to serious health problems.
Helper Turns Destroyer
Dr. Barres proposes that many adult neurodegenerative diseases are initiated when a neural injury activates an astrocyte reaction that, in turn, triggers the complement cascade. The nature of the injury is different for each disease. For example, multiple sclerosis begins with a severe autoimmune reaction, Alzheimer's disease with a buildup of misshapen proteins, and glaucoma with plumbing problems that damage the retina. In each condition, however, the result is the elimination of useful synapses and destruction of essential neural circuits.
Previous observations are consistent with this idea. For example, although the adult brain does not normally express significant levels of complement proteins, they are present in large amounts in the brains of people with neurodegenerative diseases. Individuals with Alzheimer's disease, for example, may have complement protein levels a hundred times the normal levels.
To test the theory in one neurodegenerative disease, glaucoma, the researchers collaborated with Dr. Simon John of the Howard Hughes Medical Institute's Jackson Laboratory in Bar Harbor, Maine. Using mutant mice that were specially bred to develop glaucoma, the team discovered that complement proteins begin to build up in the adult mouse retinas long before synaptic connections are destroyed and the animals begin losing vision. Moreover, by simply measuring the C1q level in each retina, the researchers determined whether mouse retinas provided to them without labels came from animals with mild, moderate, or severe glaucoma.
In work now in progress, the researchers are studying the severity of glaucoma that results when the mice bred to have the disease are crossbred with complement-deficient mice. If complement deficiency proves protective, then new drugs that block complement activity may provide a new therapy for this disease and perhaps others.
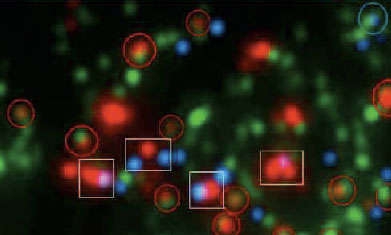 Immune Presence: The initial protein (C1q) of the immune system's complement cascade appears green in this image of the lateral geniculate nucleus (LGN). That protein tends to associate with immature synapses (circles) but not mature synapses (boxes). Mature synapses are distinguished by the close proximity of retinal nerve inputs (red) to LGN nerve processes (blue).
Immune Presence: The initial protein (C1q) of the immune system's complement cascade appears green in this image of the lateral geniculate nucleus (LGN). That protein tends to associate with immature synapses (circles) but not mature synapses (boxes). Mature synapses are distinguished by the close proximity of retinal nerve inputs (red) to LGN nerve processes (blue).Drs. Stevens and Barres hold that it might some day be possible to use measures of complement proteins to diagnose neurodegenerative diseases in their earliest stages, even before they produce symptoms. To that end, the Stanford team is looking for ways to measure C1q quantities in blood. The team is also looking for ways to block the action of C1q in order to slow or prevent the development of these diseases.
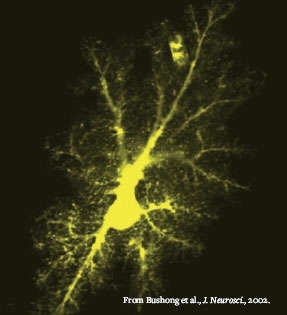 Astrocyte Aglow: This dye-filled astrocyte was imaged in the brain of a living rat. A single astrocyte interacts with five or six neurons at about 100,000 synapses.
Astrocyte Aglow: This dye-filled astrocyte was imaged in the brain of a living rat. A single astrocyte interacts with five or six neurons at about 100,000 synapses.Furthermore, Drs. Stevens and Barres are now investigating whether C1q and other complement proteins tag synapses early in a wide spectrum of neurodegenerative disorders. They are collaborating with neuropathologists who have access to human neural tissue in brain banks around the world.
Dr. Jonathan Pollock, chief of NIDA's Genetics and Molecular Neurobiology Research Branch, is enthusiastic about the research. "It's fabulous," Dr. Pollock says. "It's a significant breakthrough and helps us understand how synapses are eliminated during development. These researchers are expanding on Drs. David Hubel and Torsten Wiesel's Nobel Prize-winning work that proved that experience strengthens neural pathways that support function and eliminates those that don't. If this elimination does not take place, the brain receives too much input and can't process information properly."
Source
Stevens, B., et al. The classical complement cascade mediates CNS synapse elimination. Cell 131(6):1164-1178, 2007. [Abstract]
