People initially take cocaine for pleasure, but for most chronic abusers, the high becomes progressively shorter and weaker, and negative social and economic consequences grow increasingly dire. Relationships hit the rocks, financial problems mount, and legal trouble follows, but the cocaine abuser often fails to adapt his or her behavior to avoid the accumulating personal disasters and instead remains stuck in self-damaging patterns.
New NIDA-funded research with rats indicates that cocaine may contribute to this inflexibility by impeding abusers' ability to associate warning signs with outcomes. The research links successful sign reading to two connected brain structures—the orbitofrontal cortex (OFC), located directly above the eye sockets, and the basolateral amygdala (ABL), deep in the brain. Cocaine appears to weaken neural signaling in these structures.
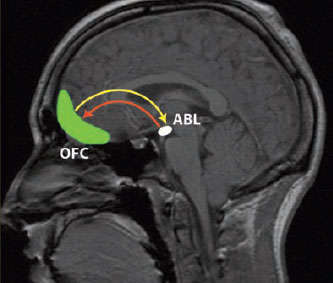 Critical Information Processing: Research indicates that cocaine weakens neural signaling in a learning circuit between the orbitofrontal cortex (OFC) and the basolateral amygdala (ABL).
Critical Information Processing: Research indicates that cocaine weakens neural signaling in a learning circuit between the orbitofrontal cortex (OFC) and the basolateral amygdala (ABL)."Our findings may explain why cocaine abusers and cocaine-exposed animals have difficulty adapting their behavior to avoid negative outcomes," says Dr. Geoffrey Schoenbaum, who led the University of Maryland School of Medicine studies. "Cocaine seems to disrupt the information-processing ability of neurons in a learning circuit that helps animals and people accommodate their behavior when the environment changes."
Learning to Use Cues
To test cocaine's impact on learning and adaptation, Dr. Schoenbaum and colleagues used a protocol called the two-odor go/no-go discrimination task (see box below). The protocol consists of two parts. The first tests an animal's ability to link cues to desired and aversive outcomes. It challenges the animal to perform a task analogous to that of a person learning that right-hand faucets deliver cold water and left-hand ones, hot. In Dr. Schoenbaum's protocol, the counterparts to the faucets are odors. The researchers give a rat a whiff of one odor immediately before filling a well in the cage with a delectable sucrose-flavored drink, and they provide another odor when the well is about to be filled with a repugnant, quinine-flavored concoction. The rats have to learn to use both cues to obtain the sweet drink and shun the nasty one.
Brain Activity Differs in Cocaine Abusers According to Gender
Cocaine abusers have reduced neural activity in the orbitofrontal cortex (OFC), a brain region that mediates decisionmaking. NIDA-funded researchers have discovered that gender determines where in the OFC the dampening occurs.
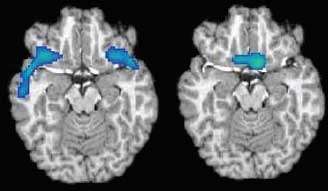 Local Lulls: Abstinent cocaine abusers show gender-specific reduction of blood flow (blue) in the OFC. Below, differences between a male brain (left) and a female brain (right).
Local Lulls: Abstinent cocaine abusers show gender-specific reduction of blood flow (blue) in the OFC. Below, differences between a male brain (left) and a female brain (right).Dr. Bryon Adinoff and colleagues at the University of Texas Southwestern Medical Center and the Veterans Affairs North Texas Health Care System measured OFC neural activity, as indicated by blood flow, of 35 people who had used cocaine for 12 years, on average, but had been abstinent for 2 to 4 weeks. They compared the results with measurements from 37 people who had never used the drug. The researchers found that the OFC contributed a smaller portion of total brain activity in cocaine abusers than in nonabusers. However, the relative deficit was in the lateral OFC in men and in the medial OFC in women.
"One can hypothesize that sex differences in regional blood flow may give rise to contrasting behavioral responses," says Dr. Adinoff. Such differences might arise because the areas most affected in each gender support different behaviors. For example, brain scans of people who do not use drugs have suggested that the lateral OFC is active when people refrain from doing something that they anticipate will have a bad outcome. In contrast, the medial OFC engages when people take action to try to achieve a desired result.
The depressed neural activity in the lateral OFC among men who abuse cocaine may lead to problems putting the brakes on behaviors with bad outcomes and so hinder their ability to abstain, says Dr. Adinoff. The less active medial area in women may reflect a blunted drug reward, he adds.
While his findings are likely to be relevant for individuals in early abstinence, Dr. Adinoff notes that they may not apply to individuals in later stages of recovery. "The participants in our study had only been abstinent 2 to 4 weeks," he says. "Scientists need to examine whether the depressed neural activity we observed among cocaine abusers recovers with long-term abstinence."
"Future research might examine whether regional differences influence treatment strategies and recovery success," notes Dr. Harold Gordon of NIDA's Division of Clinical Neuroscience and Behavioral Research.
Dr. Adinoff concurs, suggesting that through understanding these differences, treatment providers may eventually be able to tailor gender-specific therapies that promote abstinence.
Source:
Adinoff, B., et al. Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gender Medicine 3(3):206-222, 2006. [Abstract]
The second part of the go/no-go protocol tests rats' ability to adjust when cues change their meanings. The odor that formerly indicated the sweet drink now signals the bitter one, and vice versa. This put the animals into a situation analogous to that of a person whose inattentive plumber crossed the pipes leading to a sink's faucets. A person in this predicament must quickly learn to change expectations or risk repeated scaldings.
Dr. Schoenbaum's team ran two groups of rats through the go/no-go protocol. One group had been exposed to cocaine daily for 2 weeks one month prior to the protocol, and the other was drug-free. In the first part of the protocol, both groups readily learned to discriminate between the odor cues. After a dozen trials, both groups consistently—though not unerringly—went to the well following the cue for sweet and shunned it following the cue for bitter.
Tasks Test Mental Flexibility
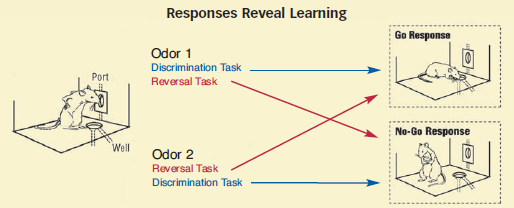
Task 1: Go/no-go discrimination
Illuminated lights indicate an odor will be forthcoming when the rat pokes its nose in the port. The odor signals which liquid, either sucrose or quinine, will appear in the well after 3 seconds. Odor 1 predicts sucrose; odor 2 predicts quinine. The rat repeatedly experiences the association between each odor cue and its taste outcome.
Interpreting the Response:
As rats learn to discriminate between the odors and use each odor's predictive significance to obtain desirable taste outcomes, they will begin to consistently head for the well when the port contains odor 1 and avoid it when the port contains odor 2.
Task 2: Reversal
Everything is the same as before, with a critical exception: The odors predict the opposite outcomes. Now, odor 1 predicts quinine; odor 2 predicts sucrose. The rat again repeatedly experiences the association between each odor cue and its taste outcome.
Interpreting the Response:
As rats learn to reverse their expectations in line with the switched predictive significance of the two odors, they will increasingly head for the well when the port contains odor 2 and avoid it when the port contains odor 1.
One observation during the first part of the protocol suggested that, despite their similar learning curves, the cocaine-exposed rats had reduced sensitivity to cues predicting negative experiences. The behavior of the rats in the two groups differed in those occasional instances where rats mistakenly went to the well following the cue for the bitter drink. The drug-naïve animals hesitated before setting off, suggesting that they had some inkling that the consequences might not be desirable. The drug-exposed animals, in contrast, rushed right to the well.
In the second part of the protocol, cocaine markedly reduced some rats' ability to adapt to the switched odor-drink pairings. The drug-naïve rats and half of the drug-exposed rats learned to reverse their responses to the cues after an average of 28 trials. The other half of the drug-exposed rats, however, required 35 trials.
Neuron Flexibility
The cocaine-exposed rats' poorer performance in the go/no-go protocols suggested that the drug impairs neurons in a brain circuit that links cues to the expectation of satisfaction or dissatisfaction. When a person or an animal responds to a cue—whether it be the position of a faucet or an odor—these neurons encode whether the experience that follows feels good or bad. In subsequent encounters with the cue, some neurons increase their firing rate if past responses led to a satisfying experience; others increase their firing rate if past responses caused aversive or disappointing outcomes.
"The firing of these neurons represents the linking of the cue to an expectation of an outcome, based on previous experience," says Dr. Schoenbaum. "We believe that at the time an animal has to decide whether or how to respond, these expectations influence its decision."
To test the hypothesis that cocaine exposure affects these outcome-expectant neurons, the research teams ran rats through go/no-go protocols while monitoring the animals' neuronal activity in two brain areas: the OFC and ABL. The OFC is part of the brain's decisionmaking circuit; its neuronal activity has been associated with stimulant addiction and craving. The ABL is part of the brain's emotional circuit. In previous studies, people with a damaged OFC or ABL were slow to change response patterns after consequences had changed from rewarding to adverse. This behavior resembles that of chronic cocaine abusers—and also some of the cocaine-exposed rats in the team's earlier experiment.
In both cocaine-exposed and unexposed rats, electrode recordings taken during the first part of the protocol showed that about 19 percent of the neurons monitored in the OFC and 26 percent of those in the ABL developed outcome-expectant firing patterns. This finding is consistent with the observation that the two groups of animals learned equally well to use the initial cues to guide their drinking.
Nevertheless, the two groups' neuronal responses may help explain why, on those occasions when the rats mistakenly responded to the quinine cue, the cocaine-exposed rats went directly to the well while the unexposed rats hesitated. The recordings revealed that the exposed rats' OFC quinine-predicting neurons failed to activate in response to the odor.
| Changed Response | Failed to Change Response | Became Nonselective | |
|---|---|---|---|
| Cocaine-exposed Rats | 15% | 27% | 58% |
| Unexposed Rats | 48% | 3% | 48% |
In the second part of the go/no-go protocol, the cocaine-exposed animals' slower adaptation correlated with reduced flexibility of outcome-expectant neurons. When the researchers reversed the odor cues, neurons predicting sweet and bitter must switch their responses to continue to support decisions leading to happy drinking experiences.
Yet approximately 27 percent of those neurons in the ABL of the drug-exposed rats failed to make the switch—compared with only approximately 3 percent in the drug-free rats. The ABL neurons of the drug-exposed rats that initially signaled favorable expectations proved more inflexible than those that signaled unfavorable expectations.
A Model for Decisionmaking
Dr. Schoenbaum's results led him to propose a model to explain how the ABL and OFC interact in cue response decisions. In this schema, when an individual encounters a familiar cue, ABL outcome-expectant neurons send the OFC a "good/go" or "bad/don't-go" message, or no message at all. The OFC combines this message with information arriving from other brain areas to form a comprehensive picture of the likely consequences of acting.
This picture becomes the basis for a decision to respond to the cue or refrain. If the individual does respond, the OFC then compares the resulting consequences with the picture and notifies the ABL whether the latest data confirm or contradict the expectation. Completing the cycle, the ABL outcome-expectant neurons use this feedback to adjust their future responses to the cue.
The key to cocaine abusers' persistent self-defeating behaviors is the drug's interference with the last step in this cycle, Dr. Schoenbaum says. Feedback from the OFC is weakened by drug exposure; consequently, ABL outcome-expectant neurons fail to change their responses. Instead, they persist in established firing patterns, continuing to signal outdated information to the OFC, and become a hindrance rather than a help to good decisionmaking.
"Cocaine renders the OFC and other frontal cortex areas' messages about likely outcomes less effective. Such signals both guide behavior and facilitate learning when things don't go as expected. By weakening the responses of these frontal cortex areas, chronic cocaine use may make people more prone to relapse and compulsive drug-seeking," says Dr. Schoenbaum.
"A person in drug abuse treatment is trying to change his or her behavior, yet these animal findings suggest that cocaine exposure ossifies a neural circuit likely involved in these changes," says Dr. Susan Volman of NIDA's Division of Basic Neuroscience and Behavioral Research. "Scientists may someday develop medications that enhance neural flexibility and facilitate reengagement of cognitive circuits, which would help behavioral therapy lessons sink in."
Dr. Elliot Stein of NIDA's Intramural Research Program, who is performing brain imaging of cocaine abusers as they do reversal learning tasks, agrees: "If the drug-exposed brain lacks plasticity for new learning, then restoring the functional integrity of the circuit may increase the effectiveness of behavioral interventions."
Sources
Stalnaker, T.A., et al. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. European Journal of Neuroscience 24(9):2643-2653, 2006. [Abstract]
Stalnaker, T.A., et al. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nature Neuroscience 10(8):949-951, 2007. [Abstract]
