Drugs of abuse trigger abrupt, massive increases in levels of the neurotransmitter dopamine that give rise to intense feelings of reward and reinforcement—the drug high—by temporarily altering the activity levels of dopamine-responsive neurons. These dopamine surges are transient, but NIDA-funded researchers recently identified a mechanism whereby they may promote long-lasting or permanent changes in behavior and cognition.
Dr. Susan George and colleagues at the University of Toronto identified a set of previously unrecognized dopamine receptors in a brain area called the striatum. When stimulated by the neurotransmitter, these receptors set in motion a cascade of events leading to a burst of calcium from stores within nerve cells. Because the cells use calcium to build and strengthen synaptic connections to other neurons, such bursts, when repeated in the setting of chronic drug abuse, can potentially rewire neural pathways that affect learning, memory, emotions, and other responses. The consequences could include the persistent symptoms and adverse effects of addiction, such as craving and reduced responsiveness to natural rewards.
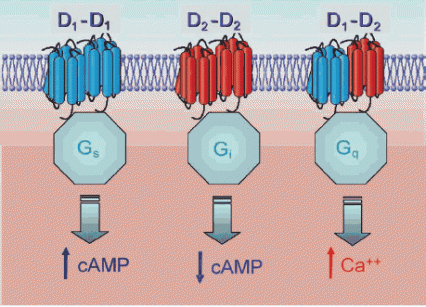 Dopamine Receptor Complexes Trigger Different Cellular Cascades: Stimulation of the D1-D2 receptor complex (on right) spurs a burst of calcium within the cell—activating the neuron, making the cell more prone to firing again, and ultimately forging new neural connections. Stimulation of the two other complexes influences slower acting biological cascades involving cyclic AMP (cAMP).
Dopamine Receptor Complexes Trigger Different Cellular Cascades: Stimulation of the D1-D2 receptor complex (on right) spurs a burst of calcium within the cell—activating the neuron, making the cell more prone to firing again, and ultimately forging new neural connections. Stimulation of the two other complexes influences slower acting biological cascades involving cyclic AMP (cAMP).Receptor Complexes Modify Cellular Responses
Although scientists had established prior to Dr. George's work that dopamine receptors can initiate calcium signaling within the brain, none of the five known dopamine receptors (D1 to D5) had been shown to do so. After searching in vain for a sixth receptor, Dr. George's team began studies that eventually led to the discovery of a set of receptors that consist of conjoined D1 and D2 receptors (see box). The researchers had previously identified other dopamine receptor complexes, including D1-D1, D2-D2, and D5-D2.
When a D1 or D2 receptor is stimulated singly, it incites a train of intracellular biochemical reactions that scientists have mapped. When both parts of a D1-D2 receptor complex are stimulated, however—something that is likely when dopamine levels surge as a result of exposure to a drug of abuse—the receptor set initiates a modified cascade that releases calcium within the cell.
Simultaneous stimulation of the two receptors in a complex results in the activation of a specialized protein, called a transducer, in the cell. The transducer acts as a molecular switch to initiate further biochemical changes. The specific transducer, called Gq, that ties activation of the D1-D2 receptor complex to the calcium cascade effects changes in gene expression, protein production, enzyme levels, the cell's firing rate, and its sensitivity to dopamine. Although scientists had already been aware of Gq, Dr. George and colleagues are the first to link it to dopamine receptors.
Complexes Expand Dopamine's Repertoire
In addition to the D1-D2 dopamine receptors, Dr. Susan George and her team at the University of Toronto have found three additional dopamine receptor complexes. Two of these consist of pairs of receptors of the same type—D1-D1 and D2-D2. Dopamine binding simultaneously to both receptors in either of these complexes activates a transducer, Gs or Gi, respectively, that ultimately stimulates or blocks synthesis of cyclic AMP, an intracellular regulatory protein (see diagram above). The other dopamine receptor complex, D5-D2, triggers a calcium burst inside the cell, like the D1-D2 but does so via a different series of biochemical interactions. D5-D2 is less likely than the D1-D2 to contribute significantly to addiction because it is not abundant in addiction-related brain areas such as the striatum.
"The direct link my colleagues and I observed between dopamine and the calcium signaling cascade suggests that drugs of abuse can rapidly and directly cause long-term changes in brain cells that respond to dopamine," says Dr. George. "The cellular mechanisms underlying these changes may provide a new window for viewing drug abuse." She adds that the D1-D2 receptor complex may also figure in schizophrenia, a disease that is thought to involve abnormal interactions between dopamine receptors and dysfunctional calcium signaling.
"To understand the full importance of the complex, researchers need to identify the specific types of neurons that express the D1-D2 complex, analyze its physiological functions, and determine how repeated activation influences the process of addiction," Dr. George says. "With such knowledge, scientists might develop compounds that selectively target the D1-D2 complex to reverse or prevent addiction or affect schizophrenia."
This work has already begun. Dr. George and her team are currently investigating the consequences for cellular form and function due to calcium signaling triggered by the D1-D2 receptor complex. Other scientists are attempting to identify compounds that selectively activate the complex. Such compounds would enable researchers to fashion radiolabeled tracers and use brain imaging to assess the quantity and distribution of the receptor complex and determine whether drugs change them. If repeated activation by drugs alters the levels or function of the complex, those changes could serve as useful markers for diagnosing drug abuse and monitoring the effects of treatment.
"The connection that Dr. George's team made between the D1-D2 complex and calcium signaling expands the range of therapeutic targets for dopamine-related neurological conditions," says Dr. David Shurtleff of NIDA's Division of Basic Neuroscience and Behavioral Research. "The emergence of D1-D2 receptor complexes over the lifespan and how they affect the progression of addiction and other neurological diseases are important areas for future research."
Sources
Rashid, A.J., et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proceedings of the National Academy of Sciences 104(2):654-659, 2007. [Full Text (PDF, 1.2MB)]
So, C.H., et al. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Molecular Pharmacology 75(4):843-854, 2009.
