One person reaches for a cigarette soon after waking, smokes a pack a day, and cannot seem to quit. Another smokes a few cigarettes now and then but never feels driven by the need for nicotine. A third person smoked for a while in youth and then stopped. According to several recent NIDA-funded studies, such contrasting smoking patterns and responses may arise because individuals inherit different forms of half a dozen genes that dictate the features of the brain receptor to which nicotine binds.
Scientists have long known that nicotine produces many of its effects by attaching to receptors for acetylcholine, a neurochemical that influences memory, arousal, attention, and mood. These nicotinic acetylcholine (nACh) receptors comprise five subunits arranged around a central pore, like sections of an orange. Each of the genes identified by the new studies provides the blueprint for one of a dozen proteins, labeled α2-10 and β2-4, that serve as subunits in nACh receptors. Variations in the DNA that encodes these genes may alter the structure or amount of the proteins produced, which in turn can modify what happens when nicotine molecules attach to the receptors.
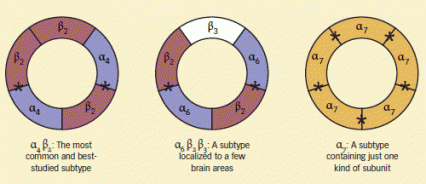 Nicotinic Receptors Vary in Component Proteins and Activity Nicotine initiates its effects by binding to nicotinic acetylcholine (nACh) receptors, each consisting of five proteins arranged in a circle around a central pore. The receptors occur in subtypes, which differ in their constituent proteins and physiological and pharmacological characteristics. Asterisks indicate where nicotine and acetylcholine bind to each receptor subtype.
Nicotinic Receptors Vary in Component Proteins and Activity Nicotine initiates its effects by binding to nicotinic acetylcholine (nACh) receptors, each consisting of five proteins arranged in a circle around a central pore. The receptors occur in subtypes, which differ in their constituent proteins and physiological and pharmacological characteristics. Asterisks indicate where nicotine and acetylcholine bind to each receptor subtype.Initially, research examining the influence of nACh receptor proteins on nicotine addiction focused on the α4 and β2 subunits. These are the most abundant and widely distributed nACh subunit proteins in the brain. Animal and human imaging studies have shown that nACh receptors consisting of two α4 and three β2 subunits are critical for the rewarding effects of nicotine.
The new studies highlight genes that code for less common nACh receptor proteins (see table below). Researchers have implicated the genes—located on chromosome 15—for the α3, α5, and β4 proteins in early initiation of smoking, the transition to dependence, and two smoking-related diseases: lung cancer and peripheral arterial disease. Investigators have also found that whether or not a person experiences extreme dizziness upon first trying cigarettes, as well as his or her risk of addiction, depends in part on the genes—on chromosome 8—for the α6 and β3 proteins. Taken together, the results suggest that genes for several nACh receptor proteins drive different aspects of the multistep process of nicotine addiction.
First Responses to Smoking
A study led by Dr. Marissa A. Ehringer at the University of Colorado examined responses of more than 1,000 17- to 21-year-olds who had been asked to recall their initial responses to smoking. All the youths had smoked almost every day for at least one month at some time, but not all had continued smoking or had become addicted to nicotine. The researchers compared the youths' reports with analysis of their DNA, focusing on the gene for the β3 protein and specifically on seven small variations called single nucleotide polymorphisms (SNPs) that produce alternate forms, or alleles, of the gene in some individuals.
Laboratory Studies Link Receptor Subtypes to Nicotine Withdrawal
Researchers are also conducting experiments with genetically engineered animals to investigate the roles of nACh proteins on smoking responses. NIDA-funded researchers Dr. M. Imad Damaj and colleagues at Virginia Commonwealth University recently showed that mice whose genes for various nACh subunit proteins had been deleted exhibited altered profiles of nicotine withdrawal. For example, removing the gene for the β2 protein reduced expressions of anxiety and aversion without affecting other signs of withdrawal; animals lacking the gene for the α7 protein, in contrast, demonstrated less hypersensitivity to pain; and deletion of the α5 protein resulted in fewer paw tremors and backing movements, which are considered to be physical signs of nicotine withdrawal. Overall, the findings suggest that the β2 nicotinic acetylcholine receptor protein contributes to the negative emotions triggered by nicotine withdrawal, while the α5 and α7 proteins underpin specific bodily signs of the withdrawal process. More recent research by Dr. Damaj and colleagues suggests that the α6 protein influences nicotine reward and negative emotions triggered by nicotine withdrawal but not acute nicotine-induced physical signs of the withdrawal process.
Sources:
Jackson, K.J. et al. The role of α6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. The Journal of Pharmacology and Experimental Therapeutics 331(2):547-554, 2009.
Jackson, K.J. et al. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. The Journal of Pharmacology and Experimental Therapeutics 325(1):302-312, 2008.
For four of the β3 SNPs, the allele that the youths possessed correlated with their initial responses to smoking, which could have included nausea, dizziness, positive feelings such as mellowness or increased energy, or negative feelings such as depression or anxiety.
The researchers looked again at two of these β3 SNPs in a followup study of a separate sample of approximately 2,500 subjects, including some sibling pairs. This complementary analysis also found evidence that these four β3 SNPs shape individuals' initial smoking responses, particularly feelings of dizziness, relaxation, or "pleasurable buzz." The followup also pointed to similar effects of one SNP in the gene for the α6 protein. More recently, the Colorado researchers, in collaboration with Dr. Laura Jean Bierut's team at Washington University in St. Louis, found an association between β3 SNPs and early response to nicotine in a third sample.
Dr Ehringer and colleagues found that the same α6 and β3 SNPs that correlated with initial responses to smoking also correlated with nicotine dependence in adults.
Dr. Ehringer hypothesizes that the intensity of early smoking experiences may be as important as their character—either pleasant or unpleasant—in determining whether someone continues to use nicotine after initial experimentation. For example, dizziness is not a sensation that people generally enjoy, yet reporting intense dizziness after the first few cigarettes is associated with nicotine dependence in adulthood.
In another project, the Colorado team tested for associations between the genes on a region of chromosome 15 that code for three nACh receptor proteins—α3, α5, and β4—and individuals' experiences with smoking and alcohol. Their initial study with youths and a larger, more focused study with adults both linked three SNPs—one in the α5 gene, one in the β4 gene, and one located between these genes—to the age at which individuals had begun using both substances. For each of these SNPs, one allele was associated with having first smoked and consumed alcohol sooner and the other with having done so later. Dr. Ehringer and colleagues hypothesize that genes in this region confer vulnerability to problematic risk-taking, a character trait that affects the age at which people begin smoking.
A genetic influence on the age of smoking initiation, such as Dr. Ehringer has identified, may have public health consequences: Studies have indicated that the younger people are when they first light up, the more likely they are to become addicted. This may be particularly true for individuals who inherit certain alleles of the gene for the α5 protein, according to Dr. Robert B. Weiss and colleagues at the University of Utah School of Medicine.
Searching for links between the genes for the α3, α5, and β4 proteins and the severity of nicotine dependence, these researchers collected smoking histories, diagnostic assessments, and DNA from over 2,800 European-American long-term smokers. The researchers found several SNPs in the α5 gene whose alternate alleles corresponded to different levels of risk for severe nicotine dependence. But the correspondence held only among people who started smoking daily before age 16. Dr. Weiss suggests that people with the higher risk alleles of these SNPs may especially benefit from early smoking prevention interventions.
| Aspect of Smoking | Subunit Gene(s) | Type of Study | Participants | Researchers |
|---|---|---|---|---|
| Dizziness from first cigarette | β3 | Gene association* | 1,075 adolescent smokers and nonsmokers | Marissa A. Ehringer et al. |
| Pleasure from initial cigarette | α5 | Gene association* | 435 adult smokers | Laura Jean Bierut, Ovide Pomerleau, Richard Sherva, et al. |
| Age of smoking initiation | α5, β4 | Gene association* | 1,075 adolescent smokers and nonsmokers | M. A. Ehringer et al. |
| Increased risk of dependence among early smokers | α5 | Candidate-gene** | 2,827 long-term smokers | Robert B. Weiss et al. |
| Transition to dependence | β3 | Genome-wide association*** | 1,929 smokers, Collaborative Genetic Study of Nicotine Dependence | L.J. Bierut, Scott Saccone, et al. |
| Transition to dependence | α3, α5, β3 | Candidate-gene** | 1,929 smokers, Collaborative Genetic Study of Nicotine Dependence | L.J. Bierut, S. Saccone, et al. |
| Transition to dependence | α5 | Genome-wide association*** | Approximately 15,000 adults | Wade Berrettini et al. |
| Transition to dependence | α3 | Genome-wide association*** | Approximately 14,000 smokers, 16,000 nonsmokers | Kári Stefánsson et al. |
| Lung cancer and peripheral arterial disease | α3 | Genome-wide association*** | Approximately 14,000 smokers, 16,000 nonsmokers | K. Stefánsson et al. |
* Links genes with smoking by comparing the genetic markers of participants with and without the condition.
** Compares genes, selected on the basis of a demonstrated or hypothesized link, from individuals with and without the condition.
*** Considers genetic markers across the entire genome to compare participants with and without the condition.
A recent study linked another aspect of risk with one of the α5 SNPs associated with nicotine dependence in the Weiss study. Drs. Bierut, Ovide Pomerleau, and colleagues found that regular smokers who had the higher-risk allele were more likely to recollect having had a pleasurable response the first time they smoked.
From the First Cigarette to Addiction
Drs. Bierut, Scott Saccone, and colleagues found that the genes for the α5 and β3 nACh receptor proteins affect an individual's risk of progressing from casual smoking to addiction. The 1,900 individuals who contributed DNA to their study had each smoked at least 100 cigarettes in their lifetime; about half were moderately to severely dependent on nicotine, while the others had not developed dependence on the drug, according to the Fagerstrom Test for Nicotine Dependence. The researchers looked for correlations between these divergent smoking histories and 4,000 SNPs within 348 genes that previous research had linked to nicotine dependence. The strongest associations were with five SNPs: two in the gene for the α3 nACh receptor subunit, two in the β3 subunit gene, and one in the α5 subunit gene. One of the α5 alleles carried double the risk of its alternative allele.
Genes Newly Linked to Nicotine Dependence
Smoking addiction commences when nicotine binds to nicotinic acetylcholine receptors, but the process ultimately involves proteins that participate in a wide variety of neuronal functions. A recent study by Dr. Laura Jean Bierut and colleagues at Washington University in St. Louis suggests that several genes that code for basic brain functions may influence an individual's vulnerability to nicotine's addictive effects.
The genome-wide association study compared the genetic profiles of a group of smokers with moderate to severe nicotine dependence to those of another group whose members had consumed at least 100 cigarettes but had not become nicotine-dependent. Out of thousands of small genetic variations examined, several were linked to genes associated with nicotine dependence. One was within a gene, called VPS13A, that helps determine which proteins should be broken down and eliminated by the waste disposal organelles inside cells. A second small variation associated with nicotine dependence was within a gene called CTNNNA3, which other researchers have linked with levels of plasma amyloid beta protein in Alzheimer's disease.
Two genes associated with calcium channels, which control the response of cells to stimulation, were also highlighted:
- CLCA, which encodes the protein that forms the calcium channel in lungs and which other research has linked to chronic obstructive pulmonary disease.
- TRPC7, which earlier work had associated with maintenance of calcium channels and with nicotine-induced behavior in roundworms.
The St. Louis researchers also corroborated a suspected association between nicotine dependence and the gene NRXN1. This gene provides the blueprint for one of a family of proteins, called the neurexins, that influence neurotransmitter release and other aspects of cell-to-cell communication. Prior research by other teams had linked genes in this family to other addictions (see "New Technique Links 89 Genes to Drug Dependence"). More recently, a NIDA-funded team led by Dr. Ming Li at the University of Virginia linked variants of the NRXN1 gene to nicotine dependence in 2,000 people from 600 families.
"This is a good start toward identifying new genes related to nicotine dependence, and the next step is to validate these findings in an independent data set and then determine what these genes may contribute to nicotine dependence," says Dr. Bierut. "The findings of our genome-wide association study, for example, point to novel biological pathways for nicotine addiction and prompt us to think of its neurobiology in a different way."
Sources:
Nussbaum, J. et al. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Human Molecular Genetics 17(11):1569-1577, 2008. [Full Text (PDF, 304KB)]
Bierut, L.J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics 16(1):24-35, 2007. [Full Text (PDF, 1.0MB)]
The trial participants who were not dependent on nicotine were unusually resistant to the drug's addictive effects, Dr. Bierut says. These are smokers who can quit at any time. Identifying the ways that the trial participants' genetic makeup protected them, even past the 100-cigarette threshold, could provide powerful clues to prevention and treatment.
"Our findings point to a role for the α3 and α5 proteins, as well as β4, in nicotine dependence," says Dr. Bierut. "The α5 proteins are expressed in the brain's reward areas, which makes our findings particularly intriguing."
Dr. Saccone says, "We are very excited to discover that genes encoding nicotinic acetylcholine receptor proteins confer risk for nicotine dependence because they have strong biological relevance to the addiction. My colleagues and I are using samples of DNA from different groups of people to confirm these findings; the next step is to determine exactly how alleles of these genes cause someone to keep smoking." Both St. Louis studies also found a link between the gene for the β3 protein—which was highlighted in the results of the Colorado studies—and nicotine dependence, bolstering confidence in this gene's involvement in the addiction.
Other researchers have corroborated and extended the St. Louis team's findings. For example, Dr. Wade Berrettini and colleagues at the University of Pennsylvania found that SNPs in the gene for the α5 protein influence the risk of smoking a pack of cigarettes a day as compared with smoking fewer than five cigarettes a day. A study led by Dr. Kári Stefánsson of deCODE Genetics, a biopharmaceutical company based in Reykjavik, Iceland, also implicated an α3 gene SNP that influences nicotine dependence but not smoking initiation. Both of these studies employed genome-wide association (GWA) scan methodology, which analyzes hundreds of thousands of SNPs simultaneously. Although GWA scans sometimes produce spurious associations, the convergence of these results with those of the St. Louis group provides strong evidence for the positive findings.
Smoking-Related Diseases
The Icelandic study included nearly 14,000 smokers, a sample large enough to detect relationships between genes and long-term smoking outcomes of lung cancer and peripheral arterial disease. Among these Caucasian European men and women, the same α3 allele that promotes dependence also accounted for 18 percent of lung cancer cases and 10 percent of peripheral arterial disease cases.
"Our results suggest that, once an individual begins smoking, this allele predisposes him or her to smoke heavily and find it difficult to stop," says Dr. Stefánsson. It may also influence other aspects of smoking, he adds, such as vulnerability to harmful effects of smoking or depth of inhalation. "These traits would affect the risk for lung cancer and peripheral arterial disease, erasing the line that people have traditionally drawn between environmental and genetic contributions," he says.
Two studies by researchers at the University of Texas in Houston and the International Agency for Research on Cancer in Lyon, France, also found that the α3 variant increases risk for lung cancer. These researchers, however, disagree with Dr. Stefánsson's explanation for the link; they argue that the variant increases cancer risk via mechanisms independent of nicotine addiction.
From Gene Discovery to Treatment
The SNPs identified in these studies may themselves affect nACh responses to nicotine by altering the form of their product proteins or their patterns of expression in different regions of the nervous system. Alternatively, further investigations may reveal that some of the SNPs are genetic bystanders that correlate with smoking behaviors only because they are inherited along with nearby, as yet unidentified DNA variants. Scientists plan to sort through these possibilities, pinpointing which alleles affect receptor responses to nicotine singly and which work in concert with others and determining how those altered responses promote or protect against nicotine addiction. A similar agenda applies to research on several genes that code for proteins that are unrelated to the nACh receptor but have recently been linked to addiction (see box below).
"Once scientists determine how these genetic variants affect nicotinic receptor function and behavioral responses to nicotine, they can develop pharmacotherapy interventions," says Dr. David Shurtleff of NIDA's Division of Basic Neuroscience and Behavioral Research. "Of the nicotinic receptors identified as novel targets in these studies, the α5 protein stands out. It seems to influence severe nicotine addiction," he says.
NIDA geneticist Dr. Joni Rutter agrees that the α5 receptor, in particular, is an interesting target. "This protein is not as abundant in the brain as other nicotinic receptor subtypes, so medications that target it might have few side effects and higher efficacies," she adds.
For people who have a genetic predisposition to various aspects of smoking addiction, the solution is simple, says Dr. Stefánsson. "It is only smoking that converts the risk into addiction and disease," he notes. "The ultimate preventive measure for these conditions is to never start smoking."
Sources
- Ehringer, M.A. et al. Association of CHRN genes with "dizziness" to tobacco. American Journal of Medical Genetics. Part B Neuropsychiatric Genetics [Epub ahead of print, September 16, 2009].
- Berrettini, W. et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry 13(4):368-373, 2008. [Full Text (PDF, 508KB)]
- Hoft, N.R. et al. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology 34(3):698-706, 2008. [Abstract]
- Schlaepfer, I.R. et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biological Psychiatry 63(11):1039-1046, 2008. [Abstract]
- Sherva, R. et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha5 (CHRNA5) with smoking status and with "pleasurable buzz" during early experimentation with smoking. Addiction 103(9):1544-1552, 2008. [Full Text (PDF, 118KB)]
- Thorgeirsson, T.E. et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452(7187):638-642, 2008. [Abstract]
- Weiss, R.B. et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genetics 4(7):e1000125, 2008. [Full Text]
- Zeiger, J.S. et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Human Molecular Genetics 17(5):724-734, 2008. [Full Text (PDF, 344KB)]
- Bierut, L.J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics 16(1):24-35, 2007. [Full Text (PDF, 1.0MB)]
- Saccone, S.F. et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. 16(1):36-49, 2007. [Full Text (PDF, 3.1MB)]
