Opioid-addicted youths benefit from extended opioid maintenance therapy, reports NIDA's Clinical Trials Network (CTN). In a study by Dr. George Woody of the Delaware Valley Node of the CTN, the Penn/VA Addiction Treatment Research Center, and the Penn Center for AIDS Research in Philadelphia, participating 15- to 21-year-olds who received drug counseling and 12 weeks of therapy with buprenorphine and naloxone abused a wide range of drugs less often than others who received only counseling and a 2-week detoxification regimen.
Although buprenorphine-naloxone is an approved medication for people age 16 and over, until now clinicians have had little research to guide them on its use in teens and young adults. A common approach, offering only short-term medication and counseling to young people, is based partly on the expectation that youths, with their shorter duration of addiction, do not require extended medication-assisted treatment, as many adults do. However, the CTN findings suggest that risk of relapse following detoxification and the potential benefit of extended buprenorphine-naloxone therapy are similar in youths and adults. The findings are timely: Rates of opioid abuse among young people have risen during the past 10 years, increasing the need for effective treatments for this population.
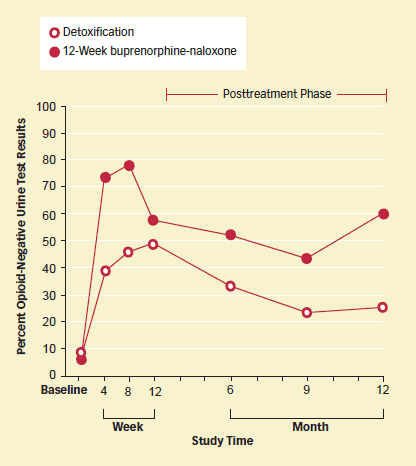 Extended Buprenorphine-Naloxone Treatment Helps Young Opioid Abusers: Opioid-addicted 15- to 21-year-olds who received counseling and continued buprenorphine-naloxone for 12 weeks with a dose taper in weeks 9-12 abused fewer opioids than others who received counseling and a 2-week detoxification. They continued to submit more opioid-negative urine specimens throughout a year of followup.
Extended Buprenorphine-Naloxone Treatment Helps Young Opioid Abusers: Opioid-addicted 15- to 21-year-olds who received counseling and continued buprenorphine-naloxone for 12 weeks with a dose taper in weeks 9-12 abused fewer opioids than others who received counseling and a 2-week detoxification. They continued to submit more opioid-negative urine specimens throughout a year of followup.The study included 152 outpatients from rural and urban community-based, CTN-affiliated treatment programs in Delaware, Maine, Maryland, New Mexico, and North Carolina. On average, the participants were 19 years old and had abused opioids for 1.5 years at the start of the study. Fifty-five percent primarily abused heroin, the majority by injection; about 35 percent primarily abused painkillers; and 10 percent abused multiple opioids. The researchers used randomization procedures to assign approximately equal numbers of participants to receive either a detoxification treatment of 2 weeks of outpatient buprenorphine-naloxone (up to 14 mg/day for 3 days, followed by a tapering of the dose) or extended treatment of 12 weeks of buprenorphine-naloxone (up to 24 mg/day for 9 weeks, followed by dose tapering that ended in week 12). All patients were scheduled to receive their clinics' standard counseling interventions in one individual session and one group session per week for 12 weeks, with more sessions available if necessary.
The impacts of the two interventions diverged quickly. At the first assessment, 2 weeks after the end of the detoxification regimen, 74 percent of the participants in the extended-maintenance group and 39 percent of those who had received only detoxification submitted opiate-free urine samples (see graph above). A similar gap continued through week 8 but narrowed to 57 percent versus 49 percent at the 12-week assessment and widened again to 60 percent versus 25 percent at the final assessment, which took place 1 year after the start of therapy. Extended therapy still yielded superior results at every assessment when the researchers tallied any missed visit as a positive urine sample. Patients in the extended therapy group also stayed in drug counseling longer, required less additional addiction treatment, reported less injection drug use, used less cocaine, and smoked less marijuana.
"The results of our study suggest that there is no hurry to stop providing buprenorphine-naloxone, an effective medication, regardless of a patient's short duration of opioid abuse," says Dr. Woody. "In my experience as a clinician, most opioid abusers—adolescent or adult—prefer to get off medication eventually. When to stop medication is an individual decision that depends on a patient's response to treatment, his or her commitment to achieving full remission without medication, and whether he or she has attained a sustained period of abstinence and a stable overall living situation."
| Percentage of Detoxification Patients | Percentage of Extended-Therapy Patients | |
|---|---|---|
| Dropped Out of Therapy | 79 | 30 |
| Abused an Opioid During the Past Week | 55 | 38 |
| Abused Marijuana During the Past Week | 26 | 16 |
| Abused Cocaine During the Past Week | 12 | 2 |
| Injected a Drug During the Past Month | 33 | 16 |
Clinicians need additional long-term evaluation of opioid addiction treatments for young people—including intensive behavioral therapy, buprenorphine-naloxone, and the opioid-blocking medication naltrexone—to identify the regimens that are most effective over the long haul, Dr. Woody says.
Dr. Betty Tai, director of NIDA's Center for Clinical Trials Network, says that Dr. Woody's findings suggest that "extended treatment with buprenorphine-naloxone is safe and effective and expands the treatment options for adolescents and young adults who are addicted to opioids, including prescription painkillers."
Source
Woody, G.E., et al. Extended vs. short-term buprenorphine-naloxone for treatment of opioid-addicted youth: A randomized trial. JAMA 300(17):2003-2011, 2008. [Full Text (PDF, 193KB)]
