Two recent NIDA-funded studies demonstrate the promise of treating addiction with medications that alleviate drug-induced alterations in signaling by the neurotransmitter glutamate. In the studies, rats treated with acetylcysteine or ceftriaxone exhibited reductions in behaviors that correspond to human relapse to cocaine and heroin abuse.
Acetylcysteine is currently prescribed to treat pulmonary disease and acetaminophen overdose, and ceftriaxone is prescribed as an antibiotic. Because their safety has been established in clinical use, addiction researchers have been able to move quickly to clinical trials. A large-scale trial with acetylcysteine is already under way.
Signal Disruption
Dr. Peter Kalivas and colleagues at the Medical University of South Carolina (MUSC) conducted the new studies. They had previously demonstrated that changes in brain glutamate signaling induced by chronic drug exposure have a wide variety of neurobiological effects that appear to be instrumental in the transition from occasional drug abuse to addiction (see inset below).
In recent work, the researchers discovered the molecular mechanism by which drugs of abuse disrupt glutamate signaling. Chronic exposure sharply reduces levels of two proteins that control the movement of glutamate into and out of non-neuronal cells called glia. Normally, the reciprocal activities of these proteins—the glial-glutamate transporter-1 (GLT-1) and the cystine-glutamate exchanger (xCT)—maintain an appropriate balance between freely circulating extracellular glutamate and glutamate sequestered inside glia. When chronic drug exposure renders GLT-1 and xCT scarce, the supply of extracellular glutamate available for neurons to use as signal molecules is diminished.
Having mapped this mechanism, the researchers identified acetylcysteine as a medication that raises the brain's production of xCT and therefore might help restore glutamate balance. Research by others suggested that ceftriaxone increases levels of GLT-1.
Levers and Learning
Dr. Kalivas and colleagues tested the medications using an animal behavioral protocol that simulates human relapse to addiction. To begin, they trained rats to press a lever to self-administer heroin or cocaine. Once the animals became steady drug takers, after about 2 weeks, the researchers deactivated the lever. Over the next 15 days in the heroin experiment (21 days in the cocaine experiment), the animals reduced the frequency with which they pressed the lever, which no longer delivered a reward. On the 16th day (22nd in the cocaine experiment), the researchers showed the animals a cue that had previously signaled drug availability. All the animals reverted to lever pressing, a response that parallels human cue-induced relapse to drug abuse.
To assess the medications' effects on drug-seeking, the researchers treated some rats in the heroin experiment with acetylcysteine, some rats in the cocaine experiment with ceftriaxone, and others with an inert substance. Each medication or inert substance was injected daily for 5 to 15 days starting on the day the lever was deactivated. The results showed that:
- The rats that received acetylcysteine learned that the lever no longer delivered heroin more quickly than the control rats. The difference was particularly striking during the first 5 days following deactivation.
- Rats that received either medication pressed the lever less often than the control animals in response to a cue or a low-dose priming injection of drug (see graph).
- The rats that received acetylcysteine also pressed the lever less often than control rats when retested 40 days after lever deactivation.
"The team's finding that acetylcysteine can attenuate rodents' drug-seeking for longer than a month is unique and astounding," says Dr. Nancy Pilotte of NIDA's Division of Basic Neuroscience and Behavioral Research. "Our results suggest that acetylcysteine may help abstinent substance abusers learn lessons from behavioral therapy more easily, as well as prevent them from relapsing. This would be a powerful double benefit for these patients," says Dr. Kalivas.
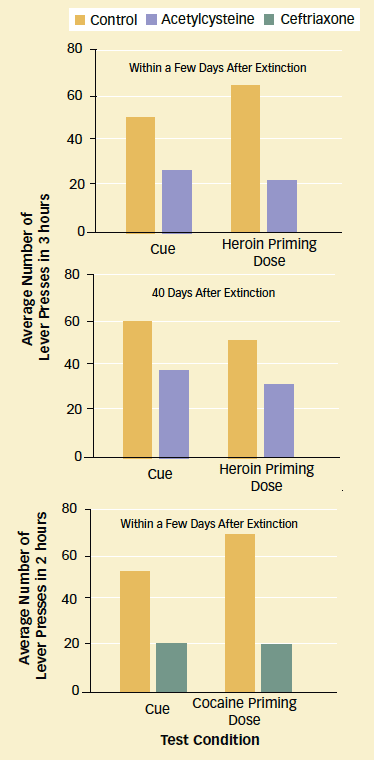 Medications Show Promise as Relapse Prevention Therapies: Rats trained to self-administer heroin or cocaine by pressing a lever gradually stop seeking the drug (extinction of drug-seeking behavior) when lever-pressing no longer results in drug delivery. After a drug-related cue or an injection of a small priming dose of the drug, the rats again press the lever, in a model of addiction relapse. However, rats that received acetylcysteine or ceftriaxone while stopping showed less heroin- and cocaine-seeking, respectively, than control animals.
Medications Show Promise as Relapse Prevention Therapies: Rats trained to self-administer heroin or cocaine by pressing a lever gradually stop seeking the drug (extinction of drug-seeking behavior) when lever-pressing no longer results in drug delivery. After a drug-related cue or an injection of a small priming dose of the drug, the rats again press the lever, in a model of addiction relapse. However, rats that received acetylcysteine or ceftriaxone while stopping showed less heroin- and cocaine-seeking, respectively, than control animals.A Mechanism Confirmed
The Kalivas group documented that, as they had predicted, xCT and GLT-1 levels were reduced in the brain tissue of experimental rats that had been exposed to heroin or cocaine, and these reductions could be reversed by treatment with acetylcysteine and ceftriaxone, respectively. The medications had no effect on those proteins in rats not exposed to drugs of abuse, suggesting acetylcysteine and ceftriaxone do not affect the glutamate balance in people who have not abused drugs.
A NIDA-funded study by Dr. George Rebec and colleagues at Indiana University yielded additional promising evidence. Following a self-administration and withdrawal protocol similar to that used by the Kalivas team, the Rebec team found that rats treated with ceftriaxone had GLT-1 levels that were twice as high in the nucleus accumbens (NAc) and three times as high in the prefrontal cortex (PFC) compared with those in rats that did not receive the medication. The increases correlated with reduced drug-seeking.
Recent experiments by Drs. Lori Knackstedt and Kalivas show that each medication influences both proteins. "Our results suggest that xCT and GLT-1 are inextricably linked and represent a crucial component of the way the healthy brain maintains the glutamate balance," says Dr. Kalivas. The link supports the idea that restoring the glutamate balance—particularly in the pathway between the PFC and NAc—might normalize drug-induced changes in this neural circuit.
Glutamate Restoration Is Linchpin of Medications' Promise
In recent years, scientists have found that as occasional drug abuse evolves into addiction, and compulsion supplants pleasure as the primary motive for drug use, glutamate rather than dopamine becomes the neurotransmitter most closely tied to drug-seeking. Dr. Peter Kalivas and colleagues at the Medical University of South Carolina (MUSC) in Charleston were among the first to call attention to glutamate as a factor in addiction some 15 years ago.
Glutamate is the brain's primary excitatory neurotransmitter, and it participates in most aspects of normal brain function. The MUSC researchers observed that glutamate signaling in key brain areas is altered when rats self-administer drugs and then undergo withdrawal. Following up on these findings, the MUSC researchers showed that chronic drug exposure upsets the balance between glutamate used as a synaptic signal between neurons and glutamate used to signal between glia and neurons. The disturbance is greatest in a neural circuit that includes the prefrontal cortex (PFC) and the nucleus accumbens (NAc), areas that affect learning, memory, and reward-seeking.
The drug-induced perturbation of glutamate signaling induces a variety of neurobiological changes that appear to influence the transition from occasional drug abuse to addiction. These include:
- Alterations in the shape and density of the tiny knob-like structures, called dendritic spines, on which neurons receive neurotransmitter signals from other neurons;
- An increase in the abundance or activity of receptors that receive glutamate signals from other neurons;
- A decrease in a receptor that limits the amount of glutamate released as a signal;
- A decrease in receptors that control neurons' ability to alter the strength of their communication in response to experience—the basic molecular mechanism of learning.
Dr. Kalivas says that the alterations in neural activity resulting from glutamate imbalance limit a chronic drug user's ability to adapt to new information—for example, to stop taking drugs in the face of adverse consequences. "Drug-induced changes in glutamate distribution strengthen the power of learned associations surrounding drugs," he says. "These associations become so strong that they take over the addicted individual's world view, obscuring the pleasure and heightening the compulsion."
Experiments by Dr. Kalivas and colleagues have implicated glutamate imbalance in the hyperresponsiveness to drug cues that is a hallmark of addiction. They showed that drug cues prompt cells in the PFC to release a surge of glutamate into the NAc of a chronically drug-exposed animal. The NAc, which has reduced extracellular glutamate, responds with heightened intensity that may trigger drug-seeking.
"Dopamine triggers reward and is critical in the early stage of addiction, but glutamate is crucial in maintaining addiction and inducing its long-term effects," says Dr. Jerry Frankenheim of NIDA's Division of Basic Neuroscience and Behavioral Research. "However, the picture is quite complex. For example, dopamine and glutamate seem to modulate each other. NIDA is supporting several researchers who are examining the dopamine-glutamate relationship."
Sources
Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nature Reviews Neuroscience 10(8):561-572, 2009. [Abstract]
Kalivas, P.W., LaLumiere, R.T., Knackstedt, L., and Shen, H. Glutamate transmission in addiction. Neuropharmacology 56(Supplement 1):169-173, 2009. [Abstract]
Moussawi, K., et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature Neuroscience 12(2):182-189, 2009. [Full Text (PDF, 1.8MB)]
Kalivas, P.W., and O'Brien, C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33(1):166-180, 2008. [Full Text]
"The team's findings suggest that glutamate is a final common pathway for drugs of abuse and highlight the therapeutic potential of medications that influence this neurochemical," says Dr. Pilotte. "These agents may help prevent relapse to many different drugs and, because glutamate does not drive the sensations of pleasure that underlie drug problems, they are likely to have low potential for abuse."
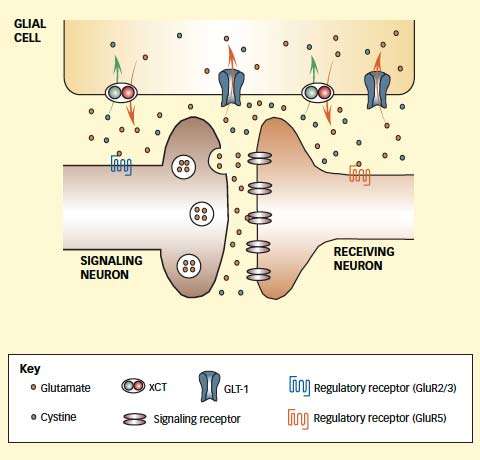 Medications Enhance Membrane Proteins That Maintain Glutamate Balance: Extracellular concentrations of glutamate in the brain affect the neurochemical's release as a signal molecule and its effect on responding neurons. Extracellular glutamate is influenced by two proteins in the membranes of glial cells: xCT pushes glutamate out of glia as it brings in the amino acid cystine, and GLT-1 carries glutamate into glial cells. Chronic drug exposure and withdrawal decrease the abundance and activity of those proteins and disrupt glutamate balance. Acetylcysteine and ceftriaxone enhance the levels and activity of these proteins. The normalized glutamate balance that results also restores activity of glutamate receptors 2/3 and 5, which influence the release of glutamate from the sending neuron and regulate the long-term effect of the signal in the receiving neuron, respectively.
Medications Enhance Membrane Proteins That Maintain Glutamate Balance: Extracellular concentrations of glutamate in the brain affect the neurochemical's release as a signal molecule and its effect on responding neurons. Extracellular glutamate is influenced by two proteins in the membranes of glial cells: xCT pushes glutamate out of glia as it brings in the amino acid cystine, and GLT-1 carries glutamate into glial cells. Chronic drug exposure and withdrawal decrease the abundance and activity of those proteins and disrupt glutamate balance. Acetylcysteine and ceftriaxone enhance the levels and activity of these proteins. The normalized glutamate balance that results also restores activity of glutamate receptors 2/3 and 5, which influence the release of glutamate from the sending neuron and regulate the long-term effect of the signal in the receiving neuron, respectively.Clinical Trials
Encouraged by the results of their animal studies, Drs. Robert Malcolm, Steven LaRowe, and Kalivas conducted pilot clinical trials. In the first, 15 cocaine abusers reported less craving, reduced desire to use, and fewer responses to visual cocaine cues when taking the acetylcysteine than when receiving a placebo. In a second study, 16 outpatients reported smoking fewer cigarettes during a 4-week regimen of acetylcysteine than in the month before the treatment. Urine tests supported their self-reports.
Dr. Kalivas' team, with MUSC colleague Dr. Malcolm, has now begun a new study with more than 200 cocaine-addicted individuals. All participants are receiving cognitive-behavioral therapy; roughly two-thirds are also getting one of two doses of acetylcysteine and the remainder, a placebo.
If the medication is effective, fewer of those in the medicated groups will relapse during the 8-week trial.
"The results of the ongoing clinical trial will be of great interest because acetylcysteine is already used clinically, and it has a known safety profile," says Dr. Pilotte. "I think that this research is promising not only for the treatment of cocaine or heroin addiction, but also to reduce dependence on other stimulants, for example, amphetamine."
Sources
Knackstedt, L.A., Melendez, R.I., and Kalivas, P.W. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological Psychiatry 67(1):81-84, 2010. [Abstract]
Sari, Y., Smith, K.D., Ali, P.K., and Rebec, G.V. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of Neuroscience 29(29):9239-9243, 2009. [Full Text (PDF, 372KB)]
Zhou, W., and Kalivas, P.W. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biological Psychiatry 63(3):338-340, 2008. [Full Text (PDF, 188KB)]
LaRowe, S.D., et al. Is cocaine desire reduced by N-acetylcysteine? American Journal of Psychiatry 164(7):1115-1117, 2007.
Mardikian, P.N., et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Progress in Neuropsychopharmacology and Biological Psychiatry 31(2):389-394, 2007. [Abstract]
