Chronic cocaine abuse may alter the production of more than 1,000 proteins in the neurons of the brain's reward system. The finding by NIDA-funded researchers sets the stage for new advances in understanding how the stimulant causes addiction. Each affected protein may contribute to the cognitive and behavioral changes that mark the transition from voluntary to compulsive drug taking and provide a lead to new anti-addiction medication strategies.
Although some of the proteins identified in the study have previously been linked to cocaine's effects, the great majority have not. Among the most intriguing of these, the researchers say, are two enzymes in the large family called sirtuins. In experiments with mice, chemically boosting the activity of these enzymes intensified drug seeking.
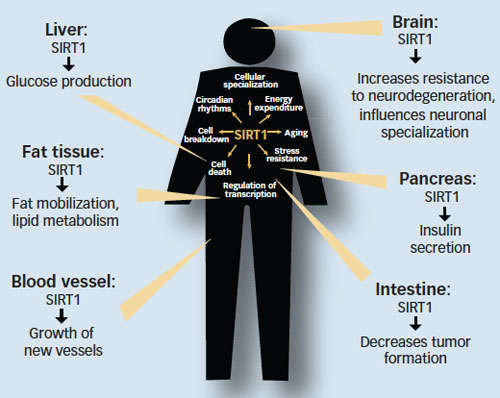 A Powerful Family of Enzymes Implicated in Cocaine's Effects: Sirtuins—also called SIRTs, for "silent information regulators of transcription"—influence a wide range of functions essential for life. This family of enzymes regulates biological processes including metabolism, DNA repair, cell specialization and death, stress resistance, and tumor growth. Sirtuin activity has been linked to longevity in worms and fruit flies. Scientists have thus far identified seven sirtuins in mammals, and SIRT1 has been studied most extensively. Cocaine regulation of sirtuin appears to be specific to one brain region.
A Powerful Family of Enzymes Implicated in Cocaine's Effects: Sirtuins—also called SIRTs, for "silent information regulators of transcription"—influence a wide range of functions essential for life. This family of enzymes regulates biological processes including metabolism, DNA repair, cell specialization and death, stress resistance, and tumor growth. Sirtuin activity has been linked to longevity in worms and fruit flies. Scientists have thus far identified seven sirtuins in mammals, and SIRT1 has been studied most extensively. Cocaine regulation of sirtuin appears to be specific to one brain region.Abundant and Suggestive Findings
Dr. Eric Nestler of Mount Sinai School of Medicine and colleagues at the University of Texas Southwestern Medical Center and Florida State University used a technique called chromatin immunoprecipitation (ChIP)-chip (see box at bottom of page) to assess cocaine's impact on the genes for 20,000 proteins in neurons of the brain area called the nucleus accumbens (NAc) of mice. The results indicated that about 5 percent of the genes were more active—likely accelerating manufacture of their protein products—following a week of exposure to cocaine, as compared with a week of exposure to saline. In a smaller—but still ample—number of other cases, cocaine reduced gene activity.
The researchers singled out a pair of sirtuins, SIRT1 and SIRT2, as being of particular interest. Sirtuins regulate basic biological processes in organisms as diverse as bacteria and humans. Although little is known about the sirtuins' function in the nervous system, their involvement in a broad range of fundamental processes suggests that the cocaine-induced increases in levels of SIRT1 and SIRT2 might play important roles in addiction.
One Protein's Dual Role in Addiction
Chronic exposure to stimulant drugs introduces many changes in the brain's reward system. Some are pathological and others appear to be countermeasures to restore neural health. Recently, Dr. Eric Nestler of Mount Sinai School of Medicine, New York, with colleagues at the University of Texas Southwestern Medical Center at Dallas revealed how one protein that has long been implicated in the development of addiction to stimulants, such as amphetamines and cocaine, also may contribute to a compensatory effect.
The protein, ΔFosB, is a transcription factor, one of a family of molecules that attach to a gene and accelerate or retard production of its protein. In previous work with animals, Dr. Nestler and others established that chronic exposure to cocaine or amphetamine causes ΔFosB to accumulate in the brain region called the striatum. This accumulation correlates with increased drug-seeking behaviors, likely by causing excesses or shortages of proteins in the nucleus accumbens and other areas of the striatum that support cognition and shape reward-related behaviors.
The net result of ΔFosB buildup in the striatum is deleterious, and scientists suspect that it may be pivotal in the transition from initial stimulant abuse to addiction. However, the effect on one protein, c-Fos, opposes amphetamine's impact.
Like ΔFosB, to which it is related, c-Fos is a transcription factor whose abundance in the striatum correlates with behavioral responses to stimulants. In contrast to ΔFosB, which responds minimally to acute drug abuse and accumulates in chronic abuse, c-Fos exhibits a different arc: Its levels rise sharply after acute stimulant exposure and wane during chronic abuse. To Dr. Nestler, these observations suggest that ΔFosB suppresses c-Fos production. The recent development of ChIP technology (see box) enabled him and his colleagues to test this proposition.
The researchers administered daily injections of amphetamine or saline to rats for a week followed by 5 days of withdrawal—a time when c-Fos reached its lowest level in striatal neurons of the animals that had received the drug. The team then used ChIP to measure ΔFosB binding to the promoter region of the gene for c-Fos. They observed that:
- More ΔFosB attached in the animals given the drug than those given saline;
- Increased amounts of bound ΔFosB correlated with reduced c-Fos production, as evidenced by lower levels of c-Fos messenger RNA;
- The ΔFosB attracted an enzyme called histone deacetylase 1 (HDAC1) that causes DNA to be held more tightly against its protein scaffolding, resulting in less production of c-Fos;
- Increasing ΔFosB in drug-naïve rats reduced the production of c-Fos in response to a single injection of amphetamine.
The researchers concluded that ΔFosB attenuates c-Fos manufacture by attracting HDAC1 to the promoter region of the c-Fos gene. "In response to repeated amphetamine exposure, brain cells mount a counter-response that reverses acute amphetamine's increased production of c-Fos," says Dr. Nestler.
Another ChIP experiment revealed a second adaptive response to chronic amphetamine exposure that reinforces the suppression of c-Fos production but is independent from that of ΔFosB. At a location in the c-Fos gene promoter region near to that where ΔFosB accumulates, an enzyme called a histone methyltransferase gathers and reduces access to DNA by causing a tightening similar to that observed with HDAC1.
"Dr. Nestler's findings point to a new pathway involving ΔFosB and represent a first step toward determining how this protein represses gene activity," says Dr. John Satterlee of NIDA's Division of Basic Neuroscience and Behavioral Research. "Moreover, they show that histone methylation—an established mechanism underlying changes in gene expression—plays a role in the brain's response to chronic amphetamine exposure."
Dr. Nestler plans to further examine how ΔFosB and c-Fos influence drug seeking and the process of addiction. He also emphasizes that ΔFosB's ability to activate some genes but inhibit others highlights the need to identify examples of both types of actions in the regulation of addictive behaviors.
Like some of the brain's other molecular responses to drugs, ΔFosB augmentation represents an exaggeration of a normal response to natural rewards such as food. "From an evolutionary perspective, accumulation of ΔFosB after natural rewards was probably adaptive," Dr. Nestler says. "It likely helped form memories for food and mates and enhanced motivation to seek these rewards again. Drugs induce an excessive amount of ΔFosB and usurp this process."
Sources
Hedges, V.L., et al. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes, Brain, and Behavior 8(4):442–449, 2009. [Full Text (PDF, 647KB)]
Renthal, W., et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. Journal of Neuroscience 28(29):7344–7349, 2008. [Full Text (PDF, 893KB)]
Wallace, D.L., et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. Journal of Neuroscience 28(41):10272–10277, 2008. [Full Text (PDF, 324KB)]
Spurred by this suggestion, Dr. Nestler and colleagues investigated the relationship between sirtuins and behavioral responses to cocaine. They administered the stimulant to mice in one chamber of a split cage until the animals began to spend most of their time there, seeking the drug. The researchers then removed the mice from the cage and infused directly into the NAc either resveratrol, a chemical that increases sirtuin activity; sirtinol, which blunts it; or an inert substance. During subsequent testing in the split cage, the resveratrol-treated mice spent twice as much time, and the sirtinol-treated animals spent half as much time, in the cocaine-associated chamber, compared with the animals in the control group. The researchers concluded that sirtuins intensify cocaine seeking in chronically exposed animals.
The results of that experiment suggested that sirtuin-inhibiting compounds might help people addicted to cocaine overcome their craving for the drug. In a preliminary test of this idea, the researchers examined whether sirtinol reduces the amount of cocaine that animals will self-administer. They infused sirtinol directly into the NAc of some rats and an inert substance into the NAc of others, then allowed the animals to self-administer cocaine by poking their noses into an aperture in a cage wall. At the dose of cocaine that showed the largest effect, the sirtinol-treated rats poked less than half as often as the control animals.
Dr. Nestler and colleagues are now investigating the molecular basis of sirtuins' influence on the brain's reward system. Experiments using brain slices show that augmenting sirtuin activity—as cocaine does by increasing the enzyme's abundance—boosts the excitability of certain neurons, an effect that is likely to heighten animals' drug seeking. Research now under way is examining the influence that these changes in neural activity might have on drug-related behaviors.
Discovery Science
The use of ChIP-chip to identify hundreds of proteins whose abundance may be altered by cocaine is an example of discovery-based science, in which researchers analyze large volumes of experimental data in search of unanticipated correlations that they can use to create new hypotheses. "My colleagues and I had not expected to see a connection between sirtuins and cocaine exposure, and we knew of no information in the scientific literature that would have predicted such alink," says Dr. Nestler. He and colleagues are also examining the consequences of altered production of many of the other proteins identified in their study.
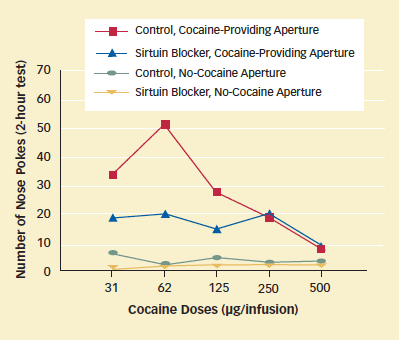 Sirtuin Blocker Reduces Rats' Motivation to Seek Cocaine: Sirtinol, a compound that inhibits sirtuin enzyme activity, decreased drug seeking among rats trained to poke their noses into an aperture to receive infusions of cocaine.
Sirtuin Blocker Reduces Rats' Motivation to Seek Cocaine: Sirtinol, a compound that inhibits sirtuin enzyme activity, decreased drug seeking among rats trained to poke their noses into an aperture to receive infusions of cocaine."Biomedical science advances when researchers better understand the mechanisms underlying illnesses," says Dr. Nestler. "Brain diseases are complex and challenging to understand, but fundamental insights into the ways that cocaine and other drugs alter the brain may lay the groundwork for the development of medications."
"The ChIP-chip technique is all about discovering new targets related to a disease or environmental exposure," says Dr. John Satterlee of NIDA's Division of Basic Neuroscience and Behavioral Research. "The discovery approach lets the organism tell investigators what is important to study, and the sirtuin findings are a great example of that approach's value to science."
ChIP and ChIP-chip at a Glance
These techniques, which can be applied to living cells, examine locations where gene-regulating proteins interact with DNA to activate or silence genes, an early step in protein production. Scientists use the methods to learn the details of gene regulation and to detect increases and decreases in activity among genes. With chromatin immunoprecipitation (ChIP), scientists examine a particular gene, whereas ChIP-on-chip (ChIP-chip) can be applied across the entire genome.
Source
Renthal, W., et al. Genome-wide analysis of chromatin regulation by cocaine reveals a novel role for sirtuins. Neuron 62(3):335–348, 2009. [Full Text (PDF, 3.4MB)]
