The MAOA gene encodes an enzyme, monoamine oxidase A, that influences fetal brain development and regulates communication in brain circuits throughout life. NIDA-funded researchers have demonstrated that the combination of prenatal smoking exposure and specific MAOA genotypes increases children's and adolescents' risk for antisocial behavior.
Intriguingly, the study found that a different genotype confers susceptibility in boys than in girls. The genotype implicated among boys is the same one that other studies have associated with antisocial behavior following another adverse exposure—childhood maltreatment—among males. Among girls, psychological tests conducted in the new study suggested that the susceptible genotype and prenatal smoking exposure together promote disruptions in social information processing.
Clues from Previous Research
In hypothesizing that the gene for the enzyme monoamine oxidase A (MAOA) underlies the link between prenatal smoking exposure and behavioral problems, Dr. Lauren Wakschlag of the Department of Medical Social Sciences at Northwestern University in Chicago, Illinois, and colleagues at several institutions drew upon previous findings. Research originally conducted by Drs. Avshalom Caspi and Terrie Moffit and colleagues, and since replicated in independent studies, had demonstrated that among males who experience maltreatment as children, those with the low-activity (MAOA-L) genotype are more likely to develop antisocial behavior than those with the high-activity (MAOA-H) genotype. Dr. Wakschlag's team reasoned that MAOA variants might similarly modify the effects of prenatal exposure to cigarettes.
To test their hypothesis, the researchers drew on their ongoing NIDA-funded East Boston Family Study, an adolescent followup to an earlier study of pregnant women, about half of whom smoked during pregnancy. Fetal exposure to smoking byproducts had been assessed by the pregnant women's repeated self-reports and blood and urine tests for cotinine, a nicotine metabolite. Together, these data provide highly accurate estimates of fetal smoking exposure, Dr. Wakschlag says.
The first adolescent followup, performed when the children were on average 15 years old, used the Diagnostic Interview Schedule for Children (C-DISC-IV) to assess symptoms of conduct disorder. About a year later, the team repeated C-DISC-IV, collected saliva for genetic analysis, and performed the Diagnostic Assessment of Nonverbal Accuracy (DANVA) to assess how well the teens could interpret the emotions expressed in pictures of faces. Finally, another year later, C-DISC-IV was performed for a third time on all the participants.
Altogether the researchers obtained full data on 176 adolescents. Roughly half the children of each sex had the MAOA-H genotype, and half had the MAOA-L genotype. The average number of conduct disorder symptoms reported per child during the entire study ranged from 0 to 15.7, with an average of 2.3. Across the three assessments, 23 percent of the boys and 7 percent of the girls met the DSM-IV three-symptom diagnostic criteria for conduct disorder.
Sex, MAOA Variants, and Risk
As the researchers anticipated, the mothers' smoking during pregnancy increased the risk for conduct disorder only in children with particular MAOA variants. Consistent with the pattern found among maltreated males, prenatal smoking exposure increased risk for boys with MAOA-L variants, but not for those with MAOA-H variants. Among girls, the reverse was true; smoking exposure increased the risk of conduct symptoms only for those with MAOA-H variants. These relationships remained after researchers took into account harsh parenting, parents' antisocial behavior, mothers' genotypes, and other potentially influential factors.
"We've now seen that a direct exposure to smoking byproducts during fetal development interacts with the MAOA genotype to increase risk of antisocial pathways, just as has been demonstrated with postnatal adverse social exposures, such as maltreatment," Dr. Wakschlag says. "What's critical in these findings is that they highlight, once again, the complex processes by which exposures that occur even before a child's birth interact with genetic susceptibility to profoundly influence children's developmental trajectories."
Commenting on the study's divergent findings in boys and girls, Dr. Wakschlag says, "In research on antisocial behavior, females often get overlooked. But we really have to consider that there can be different risk processes and behavioral patterns for girls and boys."
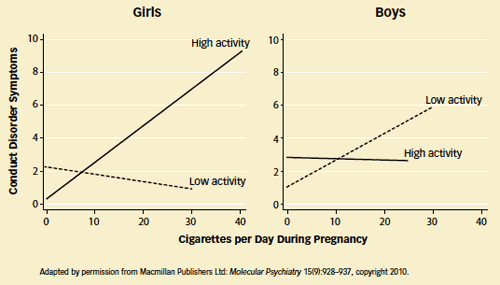 Interaction of Gene Variant and Maternal Prenatal Smoking Predicts Conduct Disorder Symptoms: Behavioral symptoms, shown here as an average score based on parental reports, are associated with the mother's intensity of smoking during pregnancy only for girls having the high-activity variant of the MAOA gene and for boys having the low-activity variant.
Interaction of Gene Variant and Maternal Prenatal Smoking Predicts Conduct Disorder Symptoms: Behavioral symptoms, shown here as an average score based on parental reports, are associated with the mother's intensity of smoking during pregnancy only for girls having the high-activity variant of the MAOA gene and for boys having the low-activity variant."There's a broad interest in sex differences in brain-behavior associations, and this field of research is just beginning," says another study author, Dr. Daniel Pine of the National Institute of Mental Health. "The first thing that people will want to do is to replicate the association we observed. Assuming that it's a consistent observation, researchers will be encouraged to explore the underlying brain mechanisms."
A More Menacing World
A computer-based test of how people recognize emotions in others gave some clues as to how the MAOA genotype and prenatal smoking exposure might alter some children's perceptions in a way that would increase disruptive behavior. Dr. Dodge and others have demonstrated that children with conduct problems often demonstrate an information-processesing style, called hostile attribution bias, in which they misperceive neutral or ambiguous situations as hostile.
In Dr. Wakschlag's study, among adolescent girls without prenatal smoking exposure, skill at identifying facial emotions was not influenced by MAOA variants. However, among girls with prenatal smoking exposure, those with MAOA-H variants tended to identify non-angry faces as angry, while girls with MAOA-L did not. The finding suggests that the combination of prenatal smoking exposure and the MAOA-H genotype biases the girls towards perceiving emotional cues as threatening. This difference was not found in boys—suggesting a fruitful avenue for further study, Dr. Wakschlag says.
"This emotion-processing bias suggests one possible mechanism by which the interaction of prenatal smoking exposure and a genetic variant increases risk of disruptive behavior. What is particularly intriguing is that this threat bias can be detected early in development, and there is some evidence that it can be altered via intervention," explains Dr. Wakschlag.
"The finding that hostile attribution is a potential mediator between the high-risk genotype and conduct disorder in girls gives us an idea of where we might target interventions," says Dr. Nicolette Borek, deputy branch chief in NIDA's Behavioral and Brain Development Branch. For these girls, training in recognizing facial expressions might be especially valuable. Further research will be required to determine information processing mechanisms by which prenatal smoking exposure and MAOA-L interact to influence boys' behavior.
Serotonin, Possibly
Divergent findings in boys and girls suggest that complex biological mechanisms underline the effects of the MAOA genotype and prenatal smoking exposure on behavior. Potential explanations include the MAOA enzyme's role as a regulator of serotonin, a neurotransmitter that guides brain circuit formation in the developing fetus. Like the MAOA-L genotype, prenatal exposure to nicotine may reduce MAOA levels, and thus serotonin levels, leading to disruption in the normal growth of circuits that shape emotion and stress.
"Both prenatal nicotine exposure and MAOA genotype have previously been linked to conduct disorders, but researchers hadn't looked at the two together," says Dr. Borek. "Dr. Wakschlag's work provides a great opportunity to link these two things." She stresses that while this association might increase vulnerability to problem behaviors, genetics and prenatal smoking do not make future conduct problems inevitable.
Dr. Borek says that it is important to replicate these results in young children. "If we're trying to understand these interactions with an eye on designing behavioral interventions, we want to know what happens with very early behavior," she says.
Dr. Wakschlag recently received NIDA funding to replicate and extend this research to early childhood. She and Dr. Kimberly Andrews Espy, a developmental cognitive neuroscientist at the University of Nebraska, Lincoln, and colleagues with expertise in molecular, statistical, and behavior genetics are conducting an intensive, preschool-age followup of children of women who participated when pregnant in a NIDA-funded study led by Dr. Espy.
The new project uses measurements of behavior and neurocognition that the researchers specifically developed for preschoolers. In addition to employing two complementary genetic approaches, the study will apply measures of brain reactivity to subgroups of exposed and nonexposed children. Dr. Espy says, "We hope to explicate the complex pathways from genes to brain to behavior and gain a much more precise understanding of the impact of prenatal tobacco exposure across development. The study design will allow us to elucidate the role of prenatal tobacco exposure in the complex and dynamic gene-environment milieu."
Source
Wakschlag, L.S., et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry 15(9):928–937, 2010. [Full Text (PDF, 406KB)]
