Three teams of NIDA-funded investigators have implicated the neuropeptide orexin (also called hypocretin) in responses that can foster the transition from casual cocaine use to regular abuse and relapse. Two of the teams also tied orexin to eating high-caloric foods that can promote obesity. The findings bode well for a strategy of treating drug abuse and overeating with medications that inhibit orexin signaling.
Working Harder For Cocaine
Dr. Stephanie Borgland of the University of British Columbia (UBC), Dr. Antonello Bonci of the University of California, San Francisco (UCSF), and colleagues demonstrated that orexin sustains cocaine seeking when access to the drug becomes difficult. Their findings suggest that orexin raises the likelihood that a person who has started using cocaine will continue—and possibly become addicted—despite mounting financial and social costs of obtaining and using the drug.
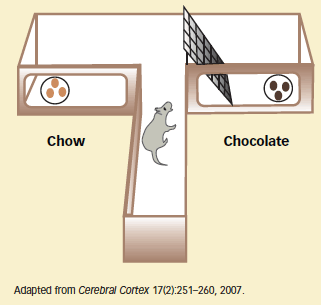 A Rat's Choice: Free Chow or Work for Chocolate? In an effort-based task, rats could obtain pellets of chow by simply walking to the end of one arm of the structure, but they had to climb over a wire-mesh barrier to get to pellets of high-fat chocolate. Most rats went over the barrier, but they did so less often when they had received SB334867, a compound that blocks receptors for the neuropeptide orexin.
A Rat's Choice: Free Chow or Work for Chocolate? In an effort-based task, rats could obtain pellets of chow by simply walking to the end of one arm of the structure, but they had to climb over a wire-mesh barrier to get to pellets of high-fat chocolate. Most rats went over the barrier, but they did so less often when they had received SB334867, a compound that blocks receptors for the neuropeptide orexin.The UBC–UCSF team conducted progressive ratio trials to investigate whether rats with normal orexin signaling will persist in cocaine seeking after rats with blocked signaling give up. At the start of a trial, animals could self-administer 0.5 mg/kg of the drug by pressing a lever once, but thereafter each dose required more presses than the previous one (see "Animal Experiments in Addiction Science"). The researchers gave some rats an inert substance and injected others with a compound, SB334867, that interrupts orexin signaling by blocking the neuropeptide from activating the orexin-1 receptor. The first group pressed the lever twice as many times as the second group before the effort needed to obtain the drug outweighed their drive for it.
Dr. Rodrigo España and colleagues at Wake Forest University (WFU) in Winston-Salem, North Carolina, independently conducted progressive ratio trials. Their results were similar to those of the UBC–UCSF team. In further experiments, the WFU team produced additional evidence that orexin comes into play as a motivator specifically when the cost of cocaine rises. They showed that:
- In a fixed ratio trial, where the work required to maintain a preferred blood concentration of cocaine remained low and constant, rats with normal and blocked orexin-1 receptors self-administered roughly equal amounts of cocaine.
- However, in a protocol that progressively escalated the amount of work necessary to maintain a preferred blood concentration, rats with normally functioning orexin receptors worked about 40 percent harder than rats with blocked receptors.
Responsiveness to Cocaine Cues
Drs. Gary Aston-Jones, Rachel Smith, Pouya Tahsil-Fahadan, and colleagues at the Medical University of South Carolina (MUSC) demonstrated that orexin increases rats' responsiveness to stimuli that accompany drug taking. Their work suggests that the neuropeptide may contribute to risk for relapse when recovering individuals encounter people, places, or things that they associate with drug use.
In one experiment, Dr. Aston-Jones and colleagues trained rats to press a lever to self-administer cocaine and to associate the experience with sound and light cues. They then deactivated the lever and cues. This phase of the protocol, called extinction training, weakened the animals' drug-lever association to the point where they stopped pressing the lever, but it left their drug-cue association intact. Finally, the researchers re-exposed the animals to the cues. Typically in experiments with addictive drugs, such re-exposure will reactivate animals' drug-lever associations and prompt them to resume avid lever pressing. In the MUSC experiment, rats with normal orexin function did just that, but animals that had been treated with SB334867 did not.
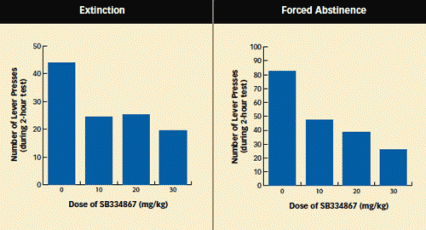 Different Models of Relapse Reveal Receptor Blocker's Effect: When returned to a chamber in which they previously self-administered cocaine, rats that had received the orexin-1 receptor blocker SB334867 sought the drug less avidly than those that had received an inert substance. Blocking orexin receptors reduced rats' drug seeking regardless of whether the animals had learned that an environment no longer offered cocaine (extinction training) or simply had not had access to the drug (forced abstinence).
Different Models of Relapse Reveal Receptor Blocker's Effect: When returned to a chamber in which they previously self-administered cocaine, rats that had received the orexin-1 receptor blocker SB334867 sought the drug less avidly than those that had received an inert substance. Blocking orexin receptors reduced rats' drug seeking regardless of whether the animals had learned that an environment no longer offered cocaine (extinction training) or simply had not had access to the drug (forced abstinence).Another MUSC experiment more closely resembled the situation of drug abusers who become abstinent outside of their home communities and then return to them. Rats self-administered cocaine in a cage with distinctive visual, auditory, olfactory, and sensory features—for example, a tone of a particular pitch, lemon scent, and mesh floor. This environment provided the animals with drug associations that had some of the richness and complexity of those experienced by drug abusers. When returned to this self-administration cage after extinction training in another cage, normal animals pressed the lever significantly more than those with blocked orexin-1 receptors.
The MUSC team designed another experiment to more closely approximate drug abusers' experience in a different respect. In contrast to animals that undergo extinction training, Dr. Aston-Jones notes, "Most drug-dependent individuals are not explicitly trained that an environment no longer offers drugs." With this in mind, the researchers trained rats to self-administer cocaine in a test cage, then moved them to another cage with no extinction training and no access to cocaine for 2 weeks. When the animals were returned to the test cage, those that were normal pressed its (now deactivated) lever 83 times in a 2-hour session, compared with 26 presses by animals whose orexin-1 receptors had been blocked with 30 mg/kg of SB334867.
One Medication For Two Compulsions?
To the UBC–UCSF and WFU researchers, their results suggested that orexin supplies the extra motivational salience that distinguishes high-impact rewards, such as cocaine provides, from normal rewards. To confirm this hypothesis, they conducted experiments with another type of substance that many people consume to the detriment of their health: delectable high-calorie food.
Drs. Borgland and Bonci and colleagues again used progressive-ratio protocols, now with either high-fat chocolate or ordinary rat chow as rewards. The results with chocolate paralleled those with the drug: Rats that received SB334867 put in less effort than rats with normal orexin signaling. In contrast, animals with normal signaling and those with blocked orexin-1 receptors worked equally hard for chow (see graph below). Similarly, when the researchers gave animals the option of climbing over a 30-cm-high wall to get chocolate or simply walking to an open bin of chow (see diagram at top of page), animals with normal orexin chose the harder path more often.
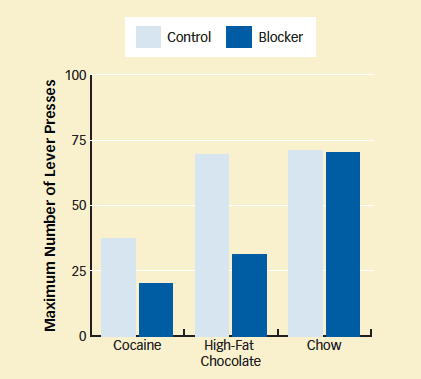 Orexin-1 Receptor Regulates Drive for Cocaine and Chocolate: Rats that received an orexin-1 receptor blocker gave up seeking high-impact rewards—cocaine and chocolate—after fewer lever presses than those in a control group that received an inert substance. The blocker did not alter rats' motivation for standard chow. In the experiments with food, rats were only slightly hungry during the test.
Orexin-1 Receptor Regulates Drive for Cocaine and Chocolate: Rats that received an orexin-1 receptor blocker gave up seeking high-impact rewards—cocaine and chocolate—after fewer lever presses than those in a control group that received an inert substance. The blocker did not alter rats' motivation for standard chow. In the experiments with food, rats were only slightly hungry during the test.Dr. Aston-Jones adds, "Patients taking an orexin-1 blocker to treat cocaine abuse would probably continue to, for example, enjoy normal food because the blocker seems to selectively influence highly salient rewards—ones with high significance either because of biological properties or through conditioning."
Dr. España and colleagues conducted an experiment that suggests that orexin is part of the answer to the question: Why are sweets in the afternoon more likely to ruin our appetite for dinner than dinner is to make us pass up dessert? They found that orexin increases animals' drive for sugar pellets when they are well-fed, but not when they are hungry (see box below).
Orexin, Appetite, and Obesity
Dr. Rodrigo España and colleagues at Wake Forest University (WFU) produced evidence suggesting that signaling at orexin-1 receptors does not have a role in eating to meet nutritional needs, but it is required for well-fed animals to seek sugar. This finding is consistent with others that have made inhibiting orexin-1 receptor signaling a candidate strategy for treating uncontrolled eating that leads to obesity.
The WFU researchers compared hungry and sated rats' willingness to work for sucrose pellets in two tests, one with normal orexin-1 receptor signaling and one after treatment with the orexin-1 receptor blocker, SB334867. The hungry animals, which had been allowed access to chow for only 1 hour per day prior to the trials, worked equally hard for sucrose pellets regardless of their orexin status. However, the sated animals, which had eaten their fill of chow prior to the trials, worked to obtain about 1.5 times as many sugar pellets when their orexin signaling was normal as they did when the neuropeptide's activity was blocked.
Source
España, R.A., et al. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. European Journal of Neuroscience 31(2):336–348, 2010. [Full Text (PDF, 2.6MB)]
Dr. Bonci, who is now scientific director of NIDA's Intramural Research Program, says that all these results encourage investigation of orexin-1 receptor blockade as a potential treatment for both cocaine addiction and overeating. "Blocking orexin-1 receptors does not appear to dampen all motivation [which would be undesirable], but it would put the brakes on drives for high-impact rewards that evoke pathological consumption."
Orexin's Neural Underpinnings
Two research teams traced orexin's motivational effects to its influence on dopamine neurons in the brain's reward pathway. Stimulation of the orexin-1 receptor, they found, mediates the superabundant dopamine release that enhances the impact of cocaine and other compulsively sought rewards. Dr. Rodrigo España and colleagues at Wake Forest University in Winston-Salem, North Carolina, demonstrated that orexin neurotransmission mediates dopamine release following cocaine exposure. After an intravenous infusion of cocaine, dopamine levels rose significantly higher in a key reward area, the nucleus accumbens (NAc), in animals with normal orexin neurotransmission than in animals with blocked orexin-1 receptors.
Dr. Stephanie Borgland of the University of British Columbia, Dr. Antonello Bonci of the University of California, San Francisco, and colleagues shed light on how orexin enhances dopamine release. They found that among rats exposed to either cocaine or high-fat food—but not less rewarding substances—dopamine neurons in the ventral tegmental area showed increased activity in response to orexin. Enhanced bursts of activity in the presence of orexin would increase dopamine release in the NAc. This work built on prior research by Dr. Gary Aston-Jones and colleagues at the Medical University of South Carolina that first reported a link between orexin and reward. They demonstrated that microinjections of orexin into the ventral tegmental area of rats induce a return to drug seeking (see "Neuropeptide Promotes Drug-Seeking and Craving in Rats").
"My colleagues and I hypothesize that when a highly salient reward is present, the orexin system kicks into play and increases dopamine release in the neurons of the reward pathway," explains Dr. Borgland. "It would follow that after administration of an orexin-1 receptor blocker, cells would be less likely to release dopamine in response to a reward—and that would make the reward less motivating."
Considering the potential use of orexin-1 blocking medications to help individuals overcome cocaine craving, Dr. España says, "Virtually all drugs of abuse cause a surge of dopamine in the brain's reward pathway, but medications that directly inhibit dopamine stifle motivation for normal behavior. Dampening dopamine indirectly with medications that block orexin receptors is a more promising strategy."
Dr. Susan Volman of NIDA's Division of Basic Neuroscience and Behavioral Research comments, "These converging findings from three laboratories suggest that signaling at orexin-1 receptors influences the ability of highly salient rewards to drive compulsive behavior. Additional research on the cellular pathways underlying orexin's effect on motivated behaviors will likely advance medication development efforts."
Sources
España, R.A., et al. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. European Journal of Neuroscience 31(2):336–348, 2010. [Full Text (PDF, 2.6MB)]
Smith, R.J.; Tahsili-Fahadan, P.; and Aston-Jones, G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology 58(1):179–184, 2010. [Full Text (PDF, 524KB)]
Borgland, S.L., et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. Journal of Neuroscience 29(36):11215–11225, 2009. [Full Text (PDF, 1.5MB)]
Smith, R.J.; See, R.E.; and Aston-Jones, G. Orexin/hypocretin signaling at the OX1 receptor regulates cue-elicited cocaine-seeking. European Journal of Neuroscience 30(3):493–503, 2009. [Full Text (PDF, 590KB)]
