Although most brain regions stop producing new neurons once the organ reaches full maturity, neurogenesis continues throughout life in the hippocampus, a structure crucial to learning and memory. Recent NIDA-funded work suggests that drugs of abuse may diminish production of new hippocampal neurons and thereby increase vulnerability to drug addiction.
Dr. Amelia J. Eisch and colleagues at the University of Texas Southwestern (UTS) Medical Center have shown that suppressing the production of new neurons in the hippocampus of adult rats increases cocaine self-administration and the persistence of cocaine-seeking behavior during withdrawal. Their results suggest that enhancing neurogenesis might be an effective strategy for treating drug abuse and preventing relapse.
A Nexus of Neurogenesis and Addiction
Dr. Eisch and collaborators hypothesized that disrupting neurogenesis in the hippocampus of laboratory rats would exacerbate the animals' drug-related behaviors. The UTS team had previously shown that cocaine and most other addictive drugs reduce adult neurogenesis (see box). Other groups had demonstrated that exercise, environmental enrichment, antidepressant medication, and some other factors that protect against drug abuse enhance neurogenesis and also that stress, a common trigger for drug abuse and relapse, depresses neurogenesis.
To test their hypothesis, Dr. Eisch and colleagues exposed rats to cranial irradiation, an experimental procedure that disrupts production of new neurons without causing illness or otherwise affecting brain function. Five weeks later, the researchers observed that the irradiated animals had only 30 percent as many new hippocampal cells as a group that was not irradiated.
A behavioral trial with the animals linked suppressed neurogenesis with increased sensitivity to the reinforcing properties of cocaine. The team placed irradiated and control rats in a chamber with a lever that the animals could press to self-administer cocaine for 4 hours daily. On the first and second days, the irradiated rats pressed the lever approximately 60 percent more often than the controls, and their frequency of self-administration remained consistently higher throughout 15 days of testing. In subsequent trials, the researchers observed similar increases in irradiated rats' self-administration of four doses of cocaine. In another experiment, rats with suppressed neurogenesis pressed the lever many more times than the control animals to obtain a single infusion of cocaine.
Before concluding that inhibited neurogenesis was the cause of the irradiated animals' increased motivation to seek cocaine, Dr. Eisch and colleagues performed cross-checks to rule out more general explanations. These experiments showed that the irradiated rats' behavior did not reflect:
- heightened sensitivity to rewards of all sorts, because animals in both groups self-administered a similar number of sucrose pellets; or
- locomotor stimulation, because rats in the two groups had similar activity levels.
Drug-Induced Disruption in Adult Neurogenesis
In studies with rats, Dr. Amelia J. Eisch and colleagues at the University of Texas Southwestern Medical Center determined the nature of the drug-induced disturbances in adult hippocampal neurogenesis and the developmental stages at which they occurred:
- Self-administered cocaine and methamphetamine inhibit adult neurogenesis at the earliest and most studied stage, called proliferation, during which cells actively divide.
- Cocaine self-administration also interferes with cell maturation, increasing the number of immature neurons in the hippocampus. After cocaine was no longer available, rats that had previously self-administered the drug showed signs of enhanced maturity in new neurons, suggesting that abstinence may promote a compensatory response following drug-induced disruption of neurogenesis.
The team's findings accord well with those of prior research, which indicated that a wide range of drugs—stimulants, nicotine, alcohol, opiates, cannabinoids—depress adult hippocampal neurogenesis. "It is striking that one sees similar results among drugs with different chemical structures, behavioral effects, and neurobiological impacts," says Dr. Eisch. "This suggests that drugs influence neurogenesis by a general mechanism—perhaps by decreasing production of proteins that promote neuron growth—rather than by acting on particular receptors or transporters."
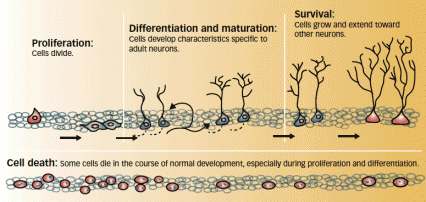 Lives of Neurons: Newly generated neurons in the adult hippocampus go through several stages of development. Drug use can affect this process during proliferation and maturation.
Lives of Neurons: Newly generated neurons in the adult hippocampus go through several stages of development. Drug use can affect this process during proliferation and maturation.Sources
Noonan, M.A., et al. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. Journal of Neuroscience 28(10):2516-2526, 2008. (Full Text)
Mandyam, C.D., et al. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis.Biological Psychiatry 64(11):958-965, 2008. (Full Text (PDF, 2.4MB))
Relapse Affected, Too
To test whether adult hippocampal neurogenesis influences vulnerability to relapse, Dr. Eisch and colleagues trained rats to self-administer cocaine, then returned the animals to their home cages with no access to the drug. After a month of withdrawal—the equivalent of an extended abstinence for a drug abuser—the rats were returned to the self-administration cages. The researchers measured how long the animals took to quit pressing the lever that had previously delivered cocaine but no longer did so. Persistence in futile drug seeking suggests vulnerability to relapse.
To the researchers' surprise, the animals' responses depended upon when hippocampal neurogenesis had been suppressed. As expected, rats irradiated at the start of the experiment pressed the lever more often during the initial self-administration period. However, following 4 weeks without cocaine, these irradiated rats quit pressing the deactivated lever just as quickly as control rats, suggesting that they were not more vulnerable to relapse. The researchers speculated that the irradiated animals' brains compensated for disrupted neurogenesis during the 12 weeks between irradiation and relapse testing.
In contrast, rats that were irradiated after cocaine self-administration showed more vulnerability to relapse than controls, taking longer to desist from pressing the lever that no longer delivered cocaine. The researchers say that this finding fits with other observations that loss of neurogenesis disrupts animals' ability to quit behaviors that no longer serve a purpose.
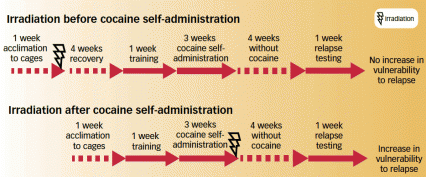 Timing Matters: Irradiation administered after—but not before—cocaine self-administration increased rats' vulnerability to relapse. Irradiation disrupts the generation of new neurons in the adult hippocampus.
Timing Matters: Irradiation administered after—but not before—cocaine self-administration increased rats' vulnerability to relapse. Irradiation disrupts the generation of new neurons in the adult hippocampus."Our results identify reduced adult hippocampal neurogenesis as a novel risk factor for addiction," says Dr. Eisch. "Moreover, our findings suggest that therapies that enhance adult neurogenesis may help prevent initial addiction as well as future relapse." While the team forges ahead with laboratory research to examine possible connections between reduced adult hippocampal neurogenesis and other drug-induced neurobiological changes, Dr. Eisch is interested in collaborating with clinicians to explore the potential of neuron production as an addiction treatment strategy.
"Although a great deal of research is needed in this area, regenerative medicine is not as far away as one might think," says Dr. Eisch. "In ongoing clinical trials, scientists are testing drugs that enhance neurogenesis as potential treatments for depression, and addiction researchers might learn valuable lessons from the results."
"If scientists can determine how adult neurogenesis is regulated and the specific function of the new cells, then they may be on the verge of developing a new type of medication to treat addiction—therapies that help the brain renew itself in a location that is compromised by drug abuse," says Dr. Nancy Pilotte of NIDA's Division of Basic Neuroscience and Behavioral Research. "The potential of this frontier area to lay a foundation for regenerative medicine is very exciting—and Dr. Eisch's research is in the vanguard."
Source
Noonan, M.A., et al. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. Journal of Neuroscience30(1):304-315, 2010. (Full Text (PDF, 1.6MB))
