NIDA-funded researchers have conducted a successful in vitro demonstration of a prototype programmable medication skin patch. As envisioned by developers Drs. Bruce Hinds and Audra Stinchcomb of the University of Kentucky, the smart patch will enable physicians to schedule transdermal medication doses that vary dynamically to match patients' fluctuating needs. Doctors in the addiction field will tailor personalized nicotine replacement and other relapseprevention therapies—for example, to program dose increases when stress, metabolic cycles, or environmental exposures may increase cravings.
Dr. Hinds, a chemist, and Dr. Stinchcomb, a research pharmacologist, developed the innovation that makes the patch possible: a membrane containing billions of carbon nanotubes, each 10,000 times thinner than a human hair. The unusual fluid flow within the carbon nanotubes allows for extremely efficient pumping; the nanotubes are voltage-gated so that they open and close to allow fluid to flow through them at rates proportional to applied electrical current. In the prototype test, the researchers steadily delivered a nicotine solution to human skin at two therapeutic dose levels.
Proof of Concept
The smart patch comprises three layers: a medication reservoir on top; the nanotube membrane in the middle; and a gel that attaches the membrane to the skin and diffuses the medication into the skin (see illustration). A watch battery that can power the pump continuously for 10 days and electronic controls complete the device, which will resemble a digital watch when fully developed.
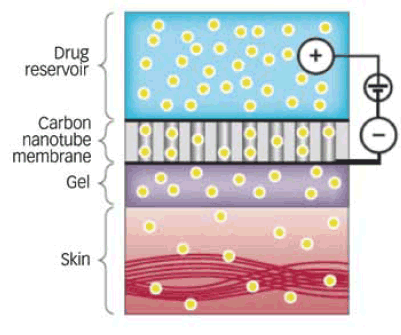 Membrane Pumps Nicotine Through Skin: When power was applied to a carbon nanotube membrane placed on a slice of gel-coated skin, the device delivered nicotine solution through the skin and into a saline bath below (not shown)
Membrane Pumps Nicotine Through Skin: When power was applied to a carbon nanotube membrane placed on a slice of gel-coated skin, the device delivered nicotine solution through the skin and into a saline bath below (not shown)In their recent demonstration, Dr. Hinds and colleagues filled the prototype reservoir with nicotine solution and applied a steady, low electrical current to the two sides of the central membrane. The gel held the device against a thin slice of human skin removed from a patient who had undergone plastic surgery for abdominal firming.
In response to the current, the membrane pumped a steady flow of nicotine through the skin into a saline bath underneath. The device delivered nicotine at two rates, both of which were consistent with therapeutic levels currently used to support smoking cessation. When the researchers shut off the power, the flow of nicotine dropped to a residual amount. No irritation of the skin was observed. "Although there are concerns about inserting carbon nanotubes into the body, our membrane is applied externally and the nanotubes are embedded in a polymer, so it is very safe," says Dr. Hinds.
The Kentucky team is now performing animal tests to lay the foundation for clinical studies. The results will show whether a smart patch powered by a watch battery can deliver therapeutic levels of nicotine into the bloodstream of guinea pigs. Dr. Hinds says that additional animal studies will help refine the delivery devices, ensuring that they can provide nicotine at the necessary rates, are safe on active creatures, and can function for long periods.
If the animal tests are successful, the next major step will be to develop a prototype smart patch for people. For this, the team plans to use a standard watch battery and, as the reservoir component, the nicotine patch already in medical use. The researchers have also created a working prototype that is controlled by Bluetooth technology and run by a smart phone program.
Wide Applications Forseen
Transdermal patches are currently available for about 20 different medications, including addiction therapeutics, replacement hormones, pain killers, and medications for motion sickness. In these treatments, the small size and other chemical properties of the active molecules enable them to move passively across the skin. For some other medications, which do not penetrate skin, adding an array of microneedles can facilitate delivery (see "Naltrexone via Skin Patch Proves Effectiveness of New Technology"). However, with current transdermal patches or microneedle devices there is no control over the timing of medication delivery.
Individualized treatment is important in the care of chronic health problems that require close monitoring of a patient's condition. For example, combined with a feedback sensor for blood glucose levels, a device containing a carbon nanotube membrane could fine-tune insulin delivery to people with diabetes more effectively than today's continually active insulin pump. As with other transdermal applications, medications delivered by the new technique bypass potential breakdown in the gastrointestinal tract and avoid causing some liver problems.
Dr. Hinds points to nicotine replacement as an example of an anti-addiction therapy that the smart patch will enhance. "There is such a large population in need of treatment that the numerous visits to a health care provider that are required to personalize dosing with today's nicotine patches may not be economically viable. With the smart patch, patients will be able to fill out an online survey of their smoking behavior and transmit it to their physician, who will use the information to remotely program the device." Remote adjustments to the programming could fine-tune medication delivery to cover such circumstances as morning cravings, an anticipated stressor, or—eventually—diminished desire to smoke. For safety, the patch design will cap the maximum rate of drug flow and incorporate an anti-tampering feature.
Modeling Nanotubes on Natural Membranes
Smart patches are just one of many applications under development for carbon nanotubes, which were first pioneered in the early 1990s. The tiny tubes are rolled from sheets of specialized carbon molecules. Strong and flexible, they offer improvement over current materials in the making of many different products, from bicycle parts to clothes to scaffolding that can support bone formation. They are excellent conductors of heat and electricity, so engineers are also using them to improve transistors, memory circuits, batteries, and other electronic components.
Dr. Bruce Hinds and colleagues at the University of Kentucky are using carbon nanotubes to create membranes that are selectively permeable to different molecules and efficient as filters and pumps. The researchers stack billions of nanotubes in the same orientation and fill the spaces between the nanotubes with a polymer. When they apply an electrical current, the tubes open and close quickly, pumping specific molecules across the membrane, just as protein channels do in living cells. The membranes can not only deliver drugs but also act as filters, fuel cells, and biological sensors.
Source
Raichle, M.E. A paradigm shift in functional brain imaging. Journal of Neuroscience 29(41):12729-12734, 2009. (Full Text (PDF, 721KB))
Dr. Thomas Aigner of NIDA's Division of Basic Neuroscience and Behavioral Research foresees multiple ways in which the smart patch could aid efforts to reduce addiction. "High-tech control of medication release would be very useful for avoiding addiction to opioid painkillers because it would ensure that patients stay within the prescribed dose and not have access to additional amounts of the drug that might be misused," says Dr. Aigner. "In addition, individualized, finely calibrated dosing of the medications used to treat opioid withdrawal could provide optimally effective alleviation of withdrawal symptoms."
Source
Wu, J., et al. Programmable transdermal drug delivery of nicotine using carbon nanotube membranes. Proceedings of the National Academy of Sciences 107(26):11698-11702, 2010. (Full Text (PDF, 651KB))
