Since their introduction in the 1960s, drugs categorized as benzodiazepines, which include diazepam (Valium) and alprazolam (Xanax), have been widely prescribed to treat anxiety and insomnia, alcohol withdrawal, and other conditions. Although they are highly effective for their intended uses, these medications must be prescribed with caution because they can be addictive. Now, work by NIDA-funded researchers has established that benzodiazepines cause addiction in a way similar to that of opioids, cannabinoids, and the club drug gamma-hydroxybutyrate (GHB). The discovery opens the door to designing new benzodiazepines that counteract anxiety but are not addictive.
Dr. Christian Lüscher and colleagues at the University of Geneva, Switzerland, studied benzodiazepines as part of a larger project to identify the point of convergence for all neurobiological pathways to drug addiction. Their findings strongly suggest that this juncture occurs when dopamine surges in response to drug taking initiate a change in synaptic plasticity in dopamine-producing cells.
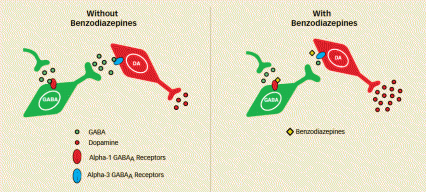 Mechanisms of Benzodiazepine Addiction (Left Image) Both inhibitory interneurons (labeled GABA) and dopaminergic neurons (labeled DA) are subject to the restraining influence of the inhibitory neurotransmitter GABA. A key difference, however, is that GABA influences the inhibitory interneurons largely via the alpha-1 subset of GABAA receptors and the dopaminergic neurons largely via the alpha-3 subtype. (Right Image) Benzodiazepines currently on the market do not interact strongly with alpha-3 GABAA receptors on dopaminergic neurons and so have no direct impact on dopamine release. However, the drugs do interact strongly with alpha-1 GABAA receptors, thereby curtailing inhibitory interneurons’ release of GABA into synapses with dopaminergic neurons. The net result is a lessening of GABA restraint on the dopaminergic neurons and an increase in dopamine release.
Mechanisms of Benzodiazepine Addiction (Left Image) Both inhibitory interneurons (labeled GABA) and dopaminergic neurons (labeled DA) are subject to the restraining influence of the inhibitory neurotransmitter GABA. A key difference, however, is that GABA influences the inhibitory interneurons largely via the alpha-1 subset of GABAA receptors and the dopaminergic neurons largely via the alpha-3 subtype. (Right Image) Benzodiazepines currently on the market do not interact strongly with alpha-3 GABAA receptors on dopaminergic neurons and so have no direct impact on dopamine release. However, the drugs do interact strongly with alpha-1 GABAA receptors, thereby curtailing inhibitory interneurons’ release of GABA into synapses with dopaminergic neurons. The net result is a lessening of GABA restraint on the dopaminergic neurons and an increase in dopamine release.
- Text description
-
This illustration provides diagrams of neurotransmitter release at synapses in the presence or absence of benzodiazapines. The first diagram shows that in the absence of benzodiazapines, GABA released from an axon of a neuron earlier in the pathway binds to an alpha-1 GABAA receptor on an inhibitory interneuron. Synapses on the axon of that interneuron then release GABA that binds to an alpha-3 GABAA receptor at a synapse of a dopamine neuron. The binding reduces that neuron’s dopamine release at its axonal synapse. The second illustration shows that benzodiazapines currently on the market bind to the alpha-1 GABAAreceptor on an inhibitory interneuron, reducing GABA binding there and subsequent GABA release by that neuron. Without the normal GABA influence, the dopamine neuron releases more dopamine than in the first diagram.
From Receptor Activation to Dopamine Surge
The pleasurable sensations that make addictive drugs disastrously attractive for vulnerable individuals occur when dopamine levels in the brain’s reward area abruptly surge. Researchers had worked out how most addictive drugs, but not benzodiazepines, precipitate these surges. Dr. Lüscher and colleagues have now demonstrated that benzodiazepines weaken the influence of a group of cells, called inhibitory interneurons, in the brain’s ventral tegmental area (VTA). These neurons normally help prevent excessive dopamine levels by downregulating the firing rates of dopamine-producing neurons. Two negatives make a positive, so when benzodiazepines limit the interneurons’ restraining influence, the dopamine-producing neurons release more dopamine.
The Swiss researchers traced benzodiazepines’ effect on VTA interneurons to the drugs’ activation of a subset of GABAA (gamma-aminobutyric acid type-A) receptors on the interneurons. Although benzodiazepines typically activate multiple subtypes of GABAA receptors, their activation of the the alpha-1 subtype is decisive for their impact on VTA interneuron behavior. These interneurons are highly sensitive to such activation because they carry abundant numbers of these receptors. By staining brain tissue, the researchers showed that 81 percent of VTA interneurons carry GABAA receptors that contain the alpha-1 subunit.
To prove that activation of alpha-1 GABAA receptors underlies benzodiazepines’ dopamine effect, the researchers administered a typical benzodiazepine, midazolam, to two groups of mice. The results supported the researchers’ proposed mechanism: In normal animals, the firing rate of interneurons decreased in response to the drug, while that of dopamine-producing neurons increased. In contrast, in animals that were genetically altered to prevent benzodiazepines from potentiating alpha-1 GABAA receptors, the drug had little or no impact on neuron firing.
A behavioral finding completed the chain of proofs linking benzodiazepines’ stimulation of alpha-1 GABAA receptors to their rewarding effects. When given the option of drinking sugar water or a sweetened solution of midazolam, normal mice imbibed roughly three times as much drug-laced as drug-free liquid. Mice with altered alpha-1 GABAA receptors, however, drank equal amounts of each, thereby exhibiting no evidence of finding one drink more rewarding than the other.
Benzodiazepines’ newly discovered mechanism for producing reward is comparable to those of opiates, cannabinoids, and GHB. Each of the four drugs reduces an inhibitory influence on dopamine-producing cells, thereby promoting dopamine spikes.
From Surge to Addiction
Dopamine surges are transient events, but addictive drugs cause long-lasting changes in the reward system. Among the earliest of these along the path from voluntary to compulsive drug use and addiction is the migration of certain AMPA receptors (i.e., GluA2-lacking receptors) from the interior to the surface of the dopamine-producing neurons. These receptors render the cell more susceptible to stimulation by the excitatory neurotransmitter glutamate, and as a result, the cells respond to future drug exposures with larger dopamine surges that produce even more intense pleasure. Scientists also have evidence that these special AMPA receptors initiate a series of changes in neural transmission that cumulatively give rise to the range of addictive symptoms.
Dr. Lüscher and colleagues showed that benzodiazepines induce AMPA receptor migration via the alpha-1 GABAA receptors. In these experiments, brain tissue from normal mice exhibited GluA2-lacking AMPA receptors after a single injection of midazolam, but tissue from mice with benzodiazepine-insensitive alpha-1 GABAA receptors did not. Recordings of intracellular electrical currents confirmed synaptic changes of dopamine-producing neurons in the normal mice and not the altered mice. To pin down the relationship further, the researchers injected mice with two other compounds, one (zolpidem) that preferentially activates only the alpha-1 GABAA receptors, and one (L-838417) that antagonizes these receptors. GluA2-lacking AMPA receptors were expressed in dopamine-producing neurons following a treatment with zolpidem, but not with L-838417.
Conclusive Proof
The Swiss researchers hypothesize that although different addictive drugs produce dopamine surges by various mechanisms, the subsequent chain of effects is the same. Consistent with this idea, they showed that even in the absence of any drug, artificial stimulation of the dopamine-producing neurons is sufficient to induce the appearance of GluA2-lacking AMPA receptors.
In this experiment, the researchers introduced a virus containing a light-activated protein, channelrhodopsin, into the dopamine-producing cells of mice. When exposed to light pulses from an optical fiber inserted into the animals’ VTA, the channelrhodopsin stimulated neuron firing in bursts similar to those produced by addictive drugs. The result was an increase in GluA2-lacking AMPA receptors comparable to that seen following exposure to addictive drugs.
“This was a nail-in-the-coffin study to show that activity of dopaminergic neurons leads to synaptic adaptation that is involved in addiction,” says Dr. Lüscher. “This is why addiction is so difficult to treat. Even if you clear the drug from the body, there are long-lasting changes in brain architecture.”
Toward Better Benzodiazepines
Taken together, the data from the studies show that the activation of alpha-1-containing GABAA receptors by benzodiazepines calms inhibitory interneurons, increasing dopaminergic neuron firing, and leads to the strengthening of excitatory synapses that favor addictions. Dr. Roger Sorensen of NIDA’s Functional Neuroscience Research Branch says, “This is the first demonstration that acute benzodiazepine use can increase dopamine release, supporting its addictive potential.”
“Now that we know that it’s the alpha-1-containing GABAA receptor that is responsible for benzodiazepine addiction, we can design benzodiazepines that do not touch those particular receptors,” says Dr. Lüscher. Drugs that bind only to alpha-2-containing GABAA receptors, he adds, might relieve anxiety nonaddictively. “Such substances already exist for research purposes,” Dr. Lüscher says. “It’s possible that we can also create them for clinical use.”
Sources
Brown, M.T.C., et al. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS One. 5:12: e15870, 2010. Full Text Available (PDF,2.2MB)
Riegel, A.C., and Kalivas, P.W. Neuroscience: Lack of inhibition leads to abuse. Nature 463: 743–744, 2010. Abstract Available
