A NIDA-supported clinical trial, the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study, has found buprenorphine to be a safe and effective alternative to methadone for treating opioid dependence during pregnancy. Women who received either medication experienced similar rates of pregnancy complications and gave birth to infants who were comparable on key indicators of neonatal health and development. Moreover, the infants born to women who received buprenorphine had milder symptoms of neonatal opioid withdrawal than those born to women who received methadone.
Methadone and buprenorphine maintenance therapy are both widely used to help individuals with opioid dependence achieve and sustain abstinence. Methadone has been the standard of care for the past 40 years for opioid-dependent pregnant women. However, interest is growing in the possible use of buprenorphine, a more recently approved medication, as another option for the treatment of opioid addiction during pregnancy.
“Our findings suggest that buprenorphine treatment during pregnancy has some advantages for infants compared with methadone and is equally safe,” says Dr. Hendrée Jones, who led the multicenter study while at the Johns Hopkins University School of Medicine and is now at RTI International.
A Rigorous Trial Design
Methadone maintenance therapy (MMT) enhances an opioid-dependent woman’s chances for a trouble-free pregnancy and a healthy baby. Compared with continued opioid abuse, MMT lowers her risk of developing infectious diseases, including hepatitis and HIV; of experiencing pregnancy complications, including spontaneous abortion and miscarriages; and of having a child with challenges including low birth weight and neurobehavioral problems.
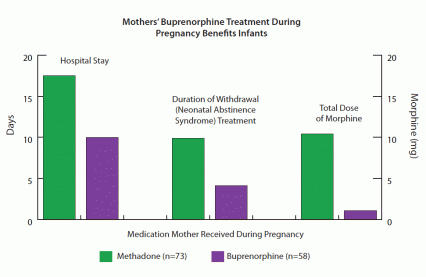
Along with these benefits, MMT may also produce a serious adverse effect. Like most drugs, methadone enters fetal circulation via the placenta. The fetus becomes dependent on the medication during gestation and typically experiences withdrawal when it separates from the placental circulation at birth. The symptoms of withdrawal, known as neonatal abstinence syndrome (NAS) include hypersensitivity and hyperirritability, tremors, vomiting, respiratory difficulties, poor sleep, and low-grade fevers. Newborns with NAS often require hospitalization and treatment, during which they receive medication (often morphine) in tapering doses to relieve their symptoms while their bodies adapt to becoming opioid-free.
The MOTHER researchers hypothesized that buprenorphine maintenance could yield methadone’s advantages for pregnant women with less neonatal distress. Buprenorphine, like methadone, reduces opioid craving and alleviates withdrawal symptoms without the safety and health risks related to acquiring and abusing drugs. Therapeutic dosing with buprenorphine, as with methadone, avoids the extreme fluctuations in opioid blood concentrations that occur in opioid abuse and place physiological stress on both the mother and the fetus. However, unlike methadone, buprenorphine is a partial rather than full opioid and so might cause less severe fetal opioid dependence than methadone therapy.
The MOTHER study recruited women as they sought treatment for opioid dependence at six treatment centers in the United States and one in Austria. All the women were 6 to 30 weeks pregnant. The research team initiated treatment with morphine for each woman, stabilized her dose, and then followed with the daily administration of buprenorphine therapy or MMT for the remainder of her pregnancy. Throughout the trial, the team increased each woman’s medication dosage as needed to ease withdrawal symptoms.
The study incorporated design features to ensure that its findings would be valid. Among the most notable were measures taken to prevent biases that might arise if staff and participants knew which medication a woman was getting.
To treat the participants without knowing which medication each woman was receiving, the study physicians wrote all prescriptions in pairs, one for each medication, in equivalent strengths. Study pharmacists matched the patient’s name and ID number to her medication group and filled only the prescription for the medication she was taking.
Each day, participants dissolved seven tablets under their tongues and then swallowed a syrup. If a woman was in the buprenorphine group, one or more of her tablets contained that medication, depending on her prescribed dosage, while the rest of the tablets and the syrup were placebos. If a woman was in the methadone group, the syrup contained that medication in her prescribed strength and the tablets all were placebos. In this way, each woman’s complement of medications appeared identical to that of every other participant. The placebo tablets and syrup were crafted to look, taste, and smell like the active medications.
As Good For Mothers, Better for Infants
Of 175 women who started a study medication, 131 continued until they gave birth. Those who received MMT and those given buprenorphine experienced similar pregnancy courses and outcomes. The two groups of women did not differ significantly in maternal weight gain, positive drug screens at birth, percentage of abnormal fetal presentations or need for Cesarean section, need for analgesia during delivery, or serious medical complications at delivery.
As the MOTHER researchers had hypothesized, the infants whose mothers were treated with buprenorphine experienced milder NAS than those infants exposed to methadone (see graph). Whereas most infants in both groups required morphine to control NAS, the buprenorphine group, on average, needed only 11 percent as much, finished its taper in less than half the time, and remained in the hospital roughly half as long as the infants exposed to methadone.
At Dr. Gabriele Fischer’s Medical University of Vienna site in Austria, three women became pregnant for a second time during the time MOTHER was enrolling participants. This development allowed researchers to compare the two medications’ relative safety and efficacy in individual women as well as across groups. During her second pregnancy, each of the three women took the alternative medication to the one she took in her first pregnancy. In each instance, the child born following buprenorphine treatment exhibited milder NAS symptoms than the one born following methadone treatment. This result suggests that differences in the effects of the two medications, rather than women’s individual differences in physiology, underlie the group findings.
“Buprenorphine may be a good option for pregnant women, particularly those who are new to treatment or who become pregnant while on this medication,” says Dr. Jones. “If a patient is on methadone maintenance and stable, however, she should remain on methadone.”
Next Questions
MOTHER researchers observed that although the women in their buprenorphine and methadone groups benefited equally from treatment, the drop-out rate was higher in the buprenorphine group (33 vs. 18 percent). This difference was not statistically significant. The researchers speculate that if it is meaningful, it may be owing to factors other than different responses to the two medications. They surmise that the experimental treatment protocols may have moved patients from morphine to buprenorphine too rapidly, causing discomfort, or that buprenorphine may have been easier than methadone to discontinue when women decided to become abstinent.
The MOTHER study did not include women with some substance use disorders that are commonly comorbid with opioid abuse. “Future studies should compare neonatal abstinence syndrome, birth outcomes, and maternal outcomes of these two medications for pregnant women who also abuse alcohol and benzodiazepines,” Dr. Jones says.
“The field also needs data on neonatal outcomes when pregnant women are treated with buprenorphine combined with naloxone, the current first-line form of buprenorphine therapy for opioid dependence,” Dr. Jones notes. The MOTHER study administered buprenorphine without naloxone to avoid exposing the fetus to a second medication with potential adverse effects.
“Research challenges remaining after this brilliant study are to determine the factors that resulted in the differential drop-out rates between the two medications,” says Dr. Loretta P. Finnegan, who did pioneering work in the assessment and treatment of NAS. “Additionally, researchers need to conduct followup research on these children to determine the longer term significance of the differences in newborn withdrawal symptoms.” Dr. Finnegan, now president of Finnegan Consulting, was formerly the medical advisor to the director of the Office of Research on Women’s Health at the National Institutes of Health.
“Neonatal abstinence syndrome is a terrible experience for infants, and there is a great need to improve care for this condition,” says Dr. Jamie Biswas of NIDA’s Division of Pharmacotherapies and Medical Consequences of Drug Abuse. “Dr. Jones’ study is a superb contribution to this area of clinical research, and the robust results should provide more treatment options for a syndrome that affects thousands of infants each year.”
Sources:
Unger, A., et al. Randomized controlled trials in pregnancy: Scientific and ethical aspects. Exposure to different opioid medications during pregnancy in an intra-individual comparison. Addiction 106(7):1355–1362, 2011. Full Text
Jones, H.E., et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. New England Journal of Medicine 363(24):2320–2331, 2010. Full Text
MOTHER Collaborators
Following is a list of collaborators on the Maternal Opioid Treatment: Human Experimental Research (MOTHER) Study and their university affiliations.
Dr. Hendrée Jones (study leader), Johns Hopkins University School of Medicine; Dr. Amelia Arria, University of Maryland, College Park; Dr. Mara Coyle, Warrant Alpert Medical School of Brown University; Dr. Gabriele Fischer, Medical University of Vienna; Dr. Sarah Heil, University of Vermont; Dr. Karol Kaltenbach, Jefferson Medical College; Dr. Peter Martin, Vanderbilt University School of Medicine; Dr. Peter Selby, University of Toronto; and Dr. Susan Stine, Wayne State University School of Medicine.