Marijuana-dependent outpatients who were treated with gabapentin in a pilot clinical trial reduced their cannabis use more and reported fewer symptoms of drug withdrawal than patients who received a placebo. Researchers Dr. Barbara Mason and colleagues at the Scripps Research Institute in La Jolla, California, suggest that their results warrant larger trials to test the medication’s potential efficacy in the broad population of individuals dependent on cannabis.
Fifty treatment-seeking adults started the 12-week trial, all of whom met DSM-IV criteria for cannabis dependence. The researchers randomly assigned 25 participants to receive gabapentin and 25 to receive placebo. All participants also received weekly counseling with motivational enhancement and cognitive behavioral techniques. Slightly more than one-third of the patients remained in treatment at the end of the trial, and the mean time in treatment among all participants was 6.4 weeks.
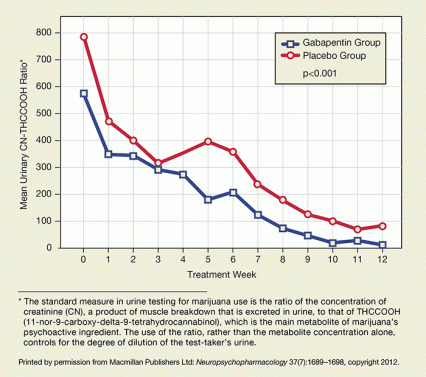 Figure 1. Gabapentin Facilitates Abstinence Patients who received gabapentin used less marijuana during treatment than did a comparison group that received placebo, according to both self-report and urinalysis.
Figure 1. Gabapentin Facilitates Abstinence Patients who received gabapentin used less marijuana during treatment than did a comparison group that received placebo, according to both self-report and urinalysis.At the study’s outset, gabapentin recipients reported ingesting 8 grams per week of marijuana on average, the placebo group 6 grams. In every subsequent week except one, gabapentin recipients reported smoking less of the drug than controls, resulting in a markedly lower total exposure. Weekly measures of THC metabolite in urine paralleled and confirmed these self-reports, with lower concentrations found in the samples of the gabapentin group at every treatment visit (see Figure 1). Both groups started the study using marijuana 5 days per week, with the gabapentin group reducing their number of days of marijuana use significantly more than the placebo group over the course of treatment. In addition, only the gabapentin group reported improvement on the Marijuana Problems Scale.
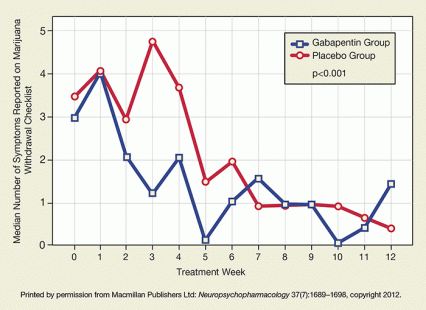 Figure 2. Gabapentin Reduced Marijuana Symptoms Compared with patients taking placebo, gabapentin patients experienced greater reductions in acute marijuana withdrawal symptoms over the course of treatment. The gabapentin patients’ symptoms had decreased by week 3, when the placebo group’s withdrawal symptoms peaked.
Figure 2. Gabapentin Reduced Marijuana Symptoms Compared with patients taking placebo, gabapentin patients experienced greater reductions in acute marijuana withdrawal symptoms over the course of treatment. The gabapentin patients’ symptoms had decreased by week 3, when the placebo group’s withdrawal symptoms peaked.Dr. Mason and colleagues hypothesize that gabapentin might help marijuana users by reducing distressing withdrawal symptoms that discourage quitting and drive relapse. In support of this idea, their gabapentin recipients reported fewer symptoms than their baseline number on the 22-item Marijuana Withdrawal Checklist in every study week after the first, while placebo recipients’ symptoms spiked in weeks 2‒4 and remained higher than the gabapentin group’s until week 7 (see Figure 2). The gabapentin group also experienced significantly less sleep disturbance, milder marijuana craving, and less depression over the course of the study.
The researchers suggest that gabapentin might improve marijuana users’ responses to behavioral treatment by reversing some of the cognitive weakening that both use of the drug and withdrawal cause. Consistent with this suggestion, 10 gabapentin recipients who underwent cognitive testing improved significantly more than 7 placebo recipients on tests of visual-motor functioning, cognitive flexibility, and ability to inhibit impulsive responses from the first to the fourth treatment week.
Gabapentin inhibits stress hormones’ impact on release of the neurotransmitter gamma aminobutyric acid (GABA) in the brain’s amygdala. This effect may contribute to alleviation of marijuana withdrawal symptoms, which arise in part because chronic marijuana abuse upsets the normal regulation of brain stress circuitry.
Physicians currently use gabapentin to treat neuropathic pain and epilepsy. NIDA funded the Scripps trial as part of its strategy to identify and test potential drug abuse treatment medications that the Food and Drug Administration has already approved for clinical use. If such a medication proves effective, it can be deployed quickly in clinical settings, whereas newly developed compounds must undergo years of preclinical and clinical testing to establish safety and efficacy.
This study was supported by NIH grants: DA020766, DA024194, and DA020766.
Source:
Mason, B.J., et al. A proof-of-concept randomized controlled study of gabapentin: Effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology 37(7):1689-1698, 2012. Abstract
