A father’s drug use can influence his child’s vulnerability to drug use and addiction by the example it sets and the environmental exposures it entails. New evidence from an animal study suggests that a father’s drug use might also affect a child’s responses to drugs even if the two never live together, or even never meet.
In the study, Dr. Fair M. Vassoler and colleagues at the University of Pennsylvania and Dr. Ghazaleh Sadri-Vakili at Massachusetts General Hospital allowed male rats to self-administer cocaine daily. After 60 days—the time it takes rat sperm cells to form—the researchers removed the rats’ access to the drug and introduced the animals to females, briefly, for mating. When the resulting pups reached young adulthood, they exhibited blunted responses to cocaine. The effect was observed even though the sires quit the drug before mating with the dams, had no contact with the dams either before or after mating with them, and never had any contact with the offspring.
The researchers trained the young-adult offspring of their cocaine-exposed rats to press a lever to self-administer cocaine infusions. The males, but not the females, took longer to build up their daily rate of lever pressing, and self-administered significantly less of the drug over 10 days, compared to control rats (see Figure 1). The control rats were offspring of rats that had never been exposed to the drug.
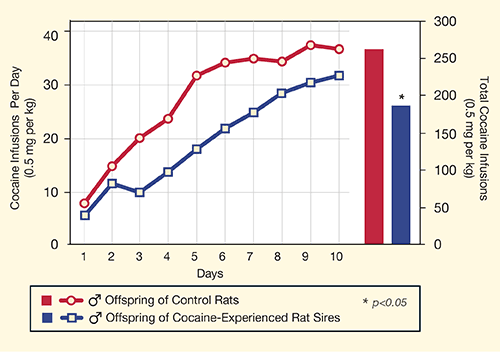 Male Offspring of Cocaine-Exposed Rats Self-Administered Less Cocaine than male offspring of rats that had not been exposed to cocaine.
Male Offspring of Cocaine-Exposed Rats Self-Administered Less Cocaine than male offspring of rats that had not been exposed to cocaine.
- Text description
-
This Figure contains two graphs that compare cocaine self-administration over 10 days by experimental rats that were offspring of cocaine-exposed rats, and control rats that were offspring of cocaine-unexposed rats. A line graph shows that the experimental rats self-administered fewer infusions than the control rats on each day. A bar graph shows that the experimental rats self-administered around 180 total infusions over the 10 days, and the control rats over 250 (p < 0.05).
Inheriting Experience
The mechanism linking the cocaine-exposed sires’ drug histories and their offspring’s behavior is epigenetic inheritance, say the researchers. The sires’ cocaine exposure induced epigenetic alterations to one or more of their genes, and the sires transmitted the alterations to their offspring via their sperm. Epigenetic alterations change the expression of a gene without changing the underlying DNA sequence. The gene produces the same protein as it did before alteration, but in greater or lesser quantities than before.
Dr. Vassoler and colleagues identified one epigenetic alteration in the sperm cells of their cocaine-exposed sires. Compared to the unexposed control sires, the cocaine-exposed sires had increased levels of acetylated histone H3 at a promoter site of the gene for brain-derived neurotrophic factor (BDNF). The added acetylation appeared to carry through to the exposed sires’ male offspring, and to raise BDNF levels in their medial frontal cortex (see Figure 2). The two groups’ contrasting BDNF levels correlated with their different levels of enthusiasm for cocaine during the self-administration trial.
Notably, paternal cocaine exposure affected cocaine self-administration by male offspring, but not female offspring. This difference suggests that hormonal effects may influence the behavior.
Dr. R. Christopher Pierce, senior researcher on the study, notes that being the child of a human parent who is addicted to cocaine heightens one’s own risk of becoming addicted. Therefore, if men with histories of cocaine use transmit the BDNF epigenetic change observed in the study to their sons, its protective effect is eclipsed by other risk factors that increase rather than reduce susceptibility to the drug. These include environmental, genetic, and possibly other epigenetic influences.
Dr. Pierce says, “Unlike our laboratory rats, drug-dependent people rarely use only one drug of abuse and often have stress, poor prenatal care, and other factors, that influence the behavior of the offspring.” Nevertheless, the finding that epigenetic inheritance can affect susceptibility to cocaine is potentially important, even if the specific inheritance observed in the study does not seem likely to play a major role in human drug abuse.
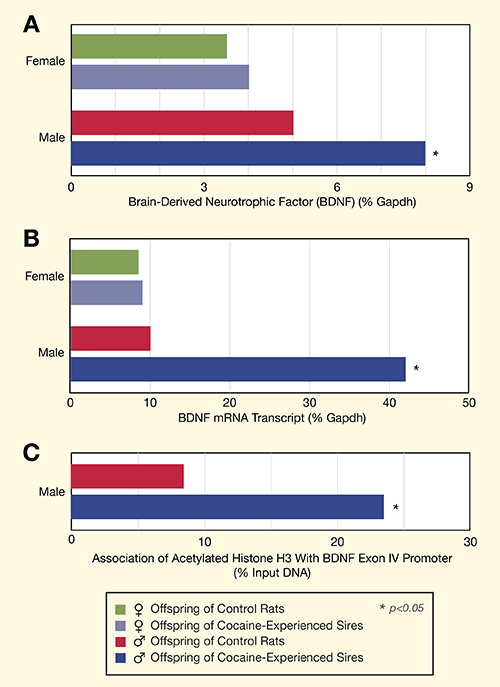 Figure 2. Brain-Derived Neurotrophic Factor Is Increased in Male Offspring of Cocaine-Exposed Sires (A) Male rats whose sires had self-administered cocaine showed increased levels of BDNF protein in the median prefrontal cortex compared with male offspring of control sires. BDNF levels did not differ significantly between female offspring of exposed and control sires. (Bars represent the ratio of BDNF to the gene for glyceraldehyde 3-phosphate dehydrogenase (%Gapdh), which is more significant for comparisons between rats than the absolute BDNF level.) (B) Male, but not female, offspring of cocaine-exposed sires had higher levels of mRNA for BDNF that contains the noncoding exon IV. (Bars represent the ratio of BDNF mRNA to the gene for glyceraldehyde 3-phosphate dehydrogenase (%Gapdh), which is more significant for comparisons between rats than the absolute BDNF mRNA level.) (C) Increased mRNA levels were associated with increased acetylation of histone H3 at the BDNF exon IV promoter. Histone acetylation is an epigenetic mechanism that promotes gene transcription and protein production.
Figure 2. Brain-Derived Neurotrophic Factor Is Increased in Male Offspring of Cocaine-Exposed Sires (A) Male rats whose sires had self-administered cocaine showed increased levels of BDNF protein in the median prefrontal cortex compared with male offspring of control sires. BDNF levels did not differ significantly between female offspring of exposed and control sires. (Bars represent the ratio of BDNF to the gene for glyceraldehyde 3-phosphate dehydrogenase (%Gapdh), which is more significant for comparisons between rats than the absolute BDNF level.) (B) Male, but not female, offspring of cocaine-exposed sires had higher levels of mRNA for BDNF that contains the noncoding exon IV. (Bars represent the ratio of BDNF mRNA to the gene for glyceraldehyde 3-phosphate dehydrogenase (%Gapdh), which is more significant for comparisons between rats than the absolute BDNF mRNA level.) (C) Increased mRNA levels were associated with increased acetylation of histone H3 at the BDNF exon IV promoter. Histone acetylation is an epigenetic mechanism that promotes gene transcription and protein production.
- Text description
-
Brain-derived Neurotrophic Factor (BDNF) (%Gapdh)
Bar chart A shows that male offspring of cocaine-experienced rat sires had significantly higher levels of BDNF, p<0.05, compared to male offspring of control rats that received no cocaine. BDNF levels of female offspring of control rats and cocaine-experience sires were similar, and both were lower than in those of either group of male offspring. Bar chart B shows that levels of BDNF mRNA transcript were four times greater in male offspring of cocaine-experience sires than in male control rats or either group of female rats, p <0.05.
Bar chart C shows that three times as much BDNF histone 3 was acetylated in offspring of cocaine-exposed rats than in offspring of control rats, p<0.05.
This study was supported by NIH grants: DA31535, DA30445, DA018678, DA18678, DA033641, DA15214, DA22339, DA33641, DA28874, and MH86599.
Source:
Vassoler, F.M. ; White, S.L. ; Schmidt, H.D. ; Sadri-Vakili, G. ; and Pierce, R.C. Epigenetic inheritance of a cocaine resistance phenotype. Nature Neuroscience 16(1):42–47, 2013. Full Text
