A lack of effective medications to treat cocaine addiction means that the current best hope for recovery is behavioral therapy, in which people learn to ignore the cues that trigger their drug craving and to establish new habits that provide healthy rewards. New research by Dr. Michael A. Nader, Dr. Robert W. Gould, and colleagues at Wake Forest School of Medicine, in Winston-Salem, North Carolina, suggests that the brain’s capacity for this type of learning is compromised during the initial days and weeks of cocaine abstinence, but may return to normal thereafter.
The Wake Forest team demonstrated that, in rhesus monkeys, ongoing cocaine exposure weakens two brain functions that people require for successful behavioral change: cognitive flexibility and memory. Similarities between the monkey and human nervous systems suggest that human cocaine users may incur similar deficits and have similar ability to recover with abstinence. The findings add to other evidence that medications that enhance cognition, such as those used to treat attention disorders, may help patients in very early abstinence profit from treatment.
Cue Switcheroo
Dr. Nader’s research team compared the cognitive performance of adult male rhesus monkeys that had self-administered cocaine almost daily for approximately 5 years and monkeys with no cocaine exposure. The monkeys were trained to sit in a chair and respond to cognitive challenges on a touch-screen device, with food rewards for success.
The first test measured the monkeys’ ability to recognize and adapt when cues change their values. This is a critical skill for patients in substance abuse treatment, who must learn to devalue drug cues and instead pursue opportunities that lead to healthy satisfactions.
The researchers showed the monkeys two shapes on a screen. Touching the correct shape earned a reward and a chance to play another round; touching the incorrect shape ended the game and chances for any further rewards. The cocaine-exposed and control monkeys took similar amounts of time to catch on to the game and start routinely touching the correct shape. At this point, the researchers reversed the cues’ significance, so that the formerly correct shape became incorrect, and vice versa. Now the impact of cocaine on cognitive flexibility appeared: Compared to the control monkeys, the drug-exposed animals took twice as long to figure out the new rules and made twice as many errors.
The researchers’ next test measured the same abilities as the first, but added complexity to more nearly approximate what patients with substance use disorders encounter in daily life. The monkeys had to learn, retain, and adjust to changes in the values of cues that were embedded in complex, potentially distracting screen displays. The displays featured two types of cues—shapes and lines—and the correct cue shifted both within and between the two. Once again, the drug-exposed animals were slower and less accurate in following the changing cue values.
Positron emission tomography (PET) scans taken within 45 minutes of the tests revealed that metabolic brain activity increased in the control monkeys, but not the cocaine-exposed animals, when the display became complicated and reward cues shifted. The areas showing differences are responsible for attention, memory, sensory integration, behavioral flexibility, stimulus-reward learning, and error detection (see Figure 1).
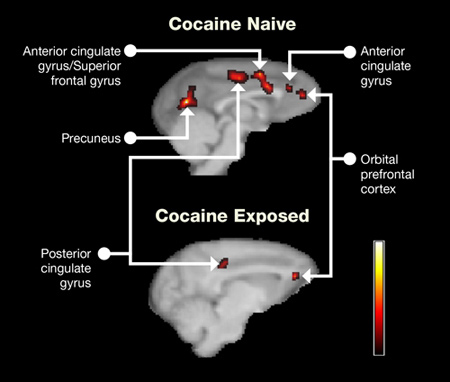
Figure 1. Cocaine Reduces Brain Activity During Learning and Memory Tasks Cocaine-exposed and control monkeys both showed high activity in the orbitofrontal cortex (OFC) during reverse-learning and set-shifting tasks. However, the cocaine-exposed monkeys showed significantly attenuated activity in the superior frontal gyrus, anterior and posterior cingulate gyrus, and precuneus. The researchers speculate that the cocaine-exposed monkeys received information normally in the OFC, but had trouble processing that information to other parts of the brain. Roll your mouse over the red areas in the PET image to read more about cognitive functions for each brain region. The scale at lower right shows metabolic activity in regions of the brain, measured by the uptake of a radioactive label. The highest activity is shown in yellow or white.
- Text description
-
PET scans of cocaine naïve and cocaine exposed rat brains show the location of metabolic activity in the brain. Areas of activity in the PET scan show in red, or in grayscale as darker grey areas bordered in black. Side views of the brain are shown. The graphic has a rollover feature so when the mouse rolls over an affected brain area, a box pops up that gives the function of that region of the brain.
Cocaine naïve rat brains showed higher activity on the PET with five areas of the brain affected. The precuneus is located midway between the center of the brain, and the back of the brain, and midway vertically within the brain. The function of this area of the brain is memory, visuospatial processing, and self-awareness. The second area, the posterior cingulate gyrus, is located above the center of the brain, situated midway front to back. This area of the brain is involved in self-awareness and working memory. The third area in the brain showing activity, including the anterior cingulate gyrus and the superior frontal gyrus, beside, but slightly below, it is located just forward of the posterior cingulate gyrus. The anterior cingulate gyrus has cognitive functions related to attention and motivation and emotional regulation. The superior frontal gyrus has higher cognitive and working memory roles. A second smaller area in the anterior cingulate gyrus further forward shows activity as well. The final area with metabolic activity is the orbital prefrontal cortex, located in the front of the brain. Its functions include decision-making, particularly evaluation of reward and punishment.
In the cocaine-exposed brain, only two areas showed activity on the PET scan, and each area was smaller than the corresponding region in the cocaine naïve rat brains. The two areas were the posterior cingulate gyrus and the orbital prefrontal cortex.
Memory Function During Binge and Recovery
People who use cocaine typically binge on the drug, taking large amounts within short time intervals between periods of abstinence or lesser use. To investigate the potential impact of cocaine bingeing, Dr. Nader and colleagues tested the monkeys’ memory while doubling the animals’ established dose of the drug for 10 days.
This test required the monkeys to learn a reward cue—a blue and yellow square, for example (see Figure 2)—and then to recognize and respond to the cue after short (a few seconds), medium (45 seconds), and long (up to 2 minutes) delays. In their first days on the binge dose, the cocaine-receiving monkeys responded more slowly to the delayed cues, and their accuracy dropped by about 25 percent, compared with their performance when on their standard dose. They managed to recover their usual speed and accuracy by the end of the 10-day period.
Something encouraging happened when the monkeys stopped receiving any cocaine and repeated the memory tests. Beginning in the third week of abstinence, their performance on medium- and long-delay tasks improved to almost 25 percent beyond what it was prior to being cut off the drug.
“These results are very promising, because they show that cocaine doesn’t have a permanent effect on cognitive performance, even after long-term use,” says Dr. Nader. “If you just get rid of the drug, our data suggest that people can recover.” He notes that 5 years of cocaine use in an animal that lives 20 to 25 years may be comparable with the experience of people who use cocaine for a decade or more.
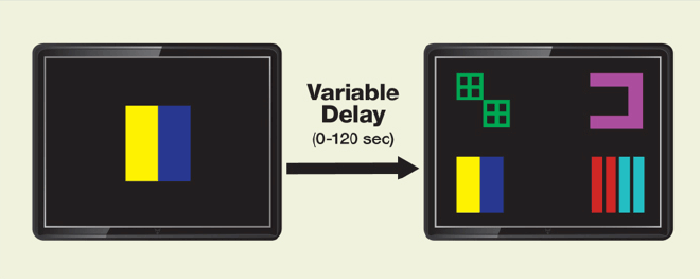 Figure 2. Touch-Screen Activities Test Monkeys’ Memory and Cognitive Flexibility Monkeys were shown the display on the left and given a treat when they touched the yellow rectangle. Once an animal learned the rule and started consistently pressing the yellow rectangle, the researchers tested its cognitive flexibility by changing the rules and rewarding touches to the blue rectangle instead. The measures of cognitive flexibility were how long and how many touches the animal required to “reverse-learn” the new rule. To test memory and cognitive flexibility in a more complex and distracting context, the researchers showed the animal the screen on the right, and recorded how long and how many tries it required to identify and touch the correct shape to receive a reward.
Figure 2. Touch-Screen Activities Test Monkeys’ Memory and Cognitive Flexibility Monkeys were shown the display on the left and given a treat when they touched the yellow rectangle. Once an animal learned the rule and started consistently pressing the yellow rectangle, the researchers tested its cognitive flexibility by changing the rules and rewarding touches to the blue rectangle instead. The measures of cognitive flexibility were how long and how many touches the animal required to “reverse-learn” the new rule. To test memory and cognitive flexibility in a more complex and distracting context, the researchers showed the animal the screen on the right, and recorded how long and how many tries it required to identify and touch the correct shape to receive a reward.Picturing Human Implications
The Wake Forest findings line up with previous observations of similar cognitive deficits in people with cocaine addiction. Because cocaine users generally use other substances as well, however, researchers have not been able to pinpoint cocaine as the sole cause of the deficits. The new findings are valuable, in large part, because they quantify the extent of the deficits and prove that they result from cocaine alone.
The fact that the rhesus monkeys’ brain scans and test performance match closely with results of similar studies in humans reinforces the value of these animals as a translational research model, says Dr. Cora Lee Wetherington of NIDA’s Behavioral and Cognitive Science Research Branch. “This model can provide a basis for developing and testing amelioration strategies, behavioral or pharmacologic, in a highly controlled, systematic manner using experimental control not afforded in human studies,” she says.
“Animal models tell us a lot that’s really relevant to the human condition,” says Dr. Nader. Repeat testing with female monkeys would be helpful, he notes, because cocaine’s effects may differ in males and females. For example, recent findings suggest that the relationship between the brain chemical dopamine and vulnerability to cocaine use may be different in male and female monkeys. It remains to be determined whether effects of cocaine on cognition, as well as potential cognitive enhancers, would also show sex differences.
This study was supported by NIH grants: DA06634, DA10584, DA006634, and DA010584.
Source:
Gould, R.W.; Gage, H.D.; Nader, M.A. Effects of chronic cocaine self-administration on cognition and cerebral glucose utilization in rhesus monkeys. Biological Psychiatry 72(10):856–63, 2012. Abstract
