More than half of heroin-addicted patients treated with naltrexone via an implanted delivery device maintained abstinence throughout a 6-month clinical trial in Saint Petersburg, Russia. The implant device, which releases a steady dose of naltrexone continuously for 2 months, averted relapse to heroin use three times as effectively as daily oral doses of the medication. Oral naltrexone, in turn, yielded a slightly, but statistically nonsignificant, higher rate of remission from heroin use than placebo.
Naltrexone is the primary medical aid to abstinence from opioid use in Russia and in some systems and treatment programs in the United States. The NIDA-supported researchers who conducted the Russian trial say that the implant device could provide clinicians and patients with a second option for extended-release naltrexone therapy, in addition to the injectable preparation (Vivitrol) that is already in clinical use. The percentage of patients who maintained unbroken abstinence with the implant in the Russian trial is similar to those reported in clinical trials of Vivitrol.
A Bulwark Against Ambivalence
Naltrexone is an opioid receptor antagonist: It blocks heroin, oxycodone, and other opioids from binding to the µ-opioid receptor, which they must do to produce their pleasurable effects. To succeed with naltrexone therapy, patients must consistently choose to take the next dose of medication rather than satisfy urges to re-experience those effects. However, naltrexone does not reliably suppress drug craving, and many patients waver in their resolve to remain abstinent. In the Russian trial as in others, more than 80 percent of patients taking the daily naltrexone pill relapsed within 6 months.
“Drug abusers are notoriously ambivalent,” says study co-leader Dr. George Woody, a professor of psychiatry at the University of Pennsylvania. “Just because they decide to quit using heroin one week doesn’t mean they’ll be motivated to quit a week later.” The rationale for extended-release naltrexone formulations is to provide sustained, long-lasting protection against such ambivalence. For example, whereas patients taking oral naltrexone must forgo the option of a pleasurable opioid experience each day, those using Vivitrol need do so only monthly.
Dr. Evgeny Krupitsky, Chief of the Laboratory of Clinical Pharmacology of Addiction at St. Petersburg Pavlov State Medical University, and Chief of the Department of Addictions at St. Petersburg Bekhterev Psychoneurological Research Institute, co-led the implant trial and managed it onsite. The trial participants, 306 heroin-addicted volunteers, underwent detoxification, then were randomly assigned to one of three groups. In two of the groups, one form of the treatment—either the implant or the pill—was active and the other a placebo; in the third group, both implant and pill were placebos. All the groups also received biweekly counseling. After 2 months, and again after 4 months, trial physicians replaced the implants, both active and placebo (see Figure). At the end of 6 months, 53 percent of the patients who received naltrexone via implant remained in treatment and had not relapsed, whereas 16 percent of those who received oral naltrexone, and 11 percent in the double-placebo group remained in treatment and had not relapsed.
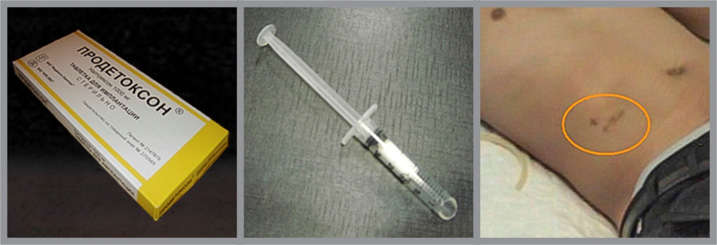 Figure. Naltrexone Implants Are Inserted Beneath the Skin of the Abdomen Surgeons inserted naltrexone and placebo implants under the skin of the abdomen, via a small incision requiring 1 to 2 stitches. The active implants contained 1,000 mg of naltrexone, which was released slowly to maintain constant plasma levels of 20 ng/mL. The implants contain a small dose of an anti-inflammatory drug and do not require removal because their matrix is made of biodegradable magnesium stearate.
Figure. Naltrexone Implants Are Inserted Beneath the Skin of the Abdomen Surgeons inserted naltrexone and placebo implants under the skin of the abdomen, via a small incision requiring 1 to 2 stitches. The active implants contained 1,000 mg of naltrexone, which was released slowly to maintain constant plasma levels of 20 ng/mL. The implants contain a small dose of an anti-inflammatory drug and do not require removal because their matrix is made of biodegradable magnesium stearate.Photos courtesy of Dr. George Woody, Department of Psychiatry, University of Pennsylvania, and Dr. Evgeny Krupitsky, Pavlov State Medical University and Bekhterev Psychoneurological Research Institute, St. Petersburg, Russian Federation.
Room for Improvement
Although the Russian trial was successful, two observations pointed to a need for further development of the naltrexone implant. First, trial participants who received the active implant developed infection and irritation at the insertion site more than four times as often as those receiving the placebo implant (9 percent versus 1 to 2 percent). All these adverse effects occurred soon after the implant was inserted and resolved promptly with antibiotic or allergy medication. The researchers believe that the implant design can be improved to avert the problems.
In addition, some 10 percent of the participants with active implants relapsed within the 2-month window during which the device is supposed to work. “It’s unclear why they relapsed,” Dr. Woody says. “It could be due to fibrosis around the implant that caused the medication to get locked in, so that it couldn’t reach the appropriate blood levels, or perhaps the medication was released too quickly and depleted too early.”
The trial results affirmed the strategy of using extended-release naltrexone to protect patients against ambivalence, but raised a question of how long the treatment should continue. By 6 months after the trial, almost all the participants, from all groups, had relapsed to heroin use. “This gives us evidence that the treatment should last longer than 6 months,” Dr. Krupitsky says.
Dr. Krupitsky has already begun to explore longer treatment. He has maintained some patients on naltrexone injections for 1 year, and says that about half have stayed off heroin the entire time, with no serious safety concerns arising.
Dr. Krupitsky notes that the St. Petersburg study did not find any evidence of patients trying to overcome the medication’s receptor blockade by taking more than their accustomed dose of the drug. Such behavior was a potential concern, because doing so would put a patient at risk for overdose when naltrexone vacated the receptors at the ends of the dosing periods.
Why Russia?
Extended-release naltrexone may fit particularly well into Russia’s system for treating heroin addiction. In that country, a large network of specialized hospitals admits patients who are seeking help for heroin abuse for at least 7 days of detoxification (See Treating Heroin Addiction in Russia below). In contrast, in the United States, says Dr. Woody, “Many people have trouble completing drug detoxification programs, sometimes because of payment or insurance issues. If people begin to take naltrexone before they have shaken off their physiological dependence on heroin, the treatment will cause abrupt and harsh symptoms of drug withdrawal.”
Another factor that makes Russia a promising ground for extended-release naltrexone is that the quality of street heroin there is generally lower than that of heroin in the United States. As a result, if patients were to try, they would find it difficult to take a high enough dose of heroin to overcome the naltrexone blockade of the opioid receptor.
Finally, naltrexone is the only medication Russian physicians currently have available to treat opiate abuse. They do not have access to the opiate agonist medications methadone and buprenorphine, or a variety of other medications that U.S. physicians can employ.
Nevertheless, although naltrexone is likely to play a less central role in heroin treatment in the United States than in Russia, opioid antagonist therapy may be the best option in numerous situations here. For example, some patients reject opioid agonist therapy, and some systems forbid the use of agonist medications. (Read an interview with Dr. Redonna Chandler on the subject of addiction treatment in the U.S. criminal justice system.)
The More Options, the Better
Clinical trials are ongoing to identify optimal patient populations and dosing schedules for naltrexone. Ultimately, Dr. Woody says, this research is not about finding a superior drug to treat opioid dependence. What works best will be different for different people and will vary according to different situations.
“Adding a new formulation of naltrexone to the arsenal of treatments for opioid addiction is a significant achievement, particularly for countries like Russia, where methadone and buprenorphine are unavailable and where the rates of HIV infection due to injection drug use continue to rise,” says Dr. Ivan Montoya, deputy director of NIDA’s Division of Pharmacotherapies and Medical Consequences of Drug Abuse.
“Methadone and buprenorphine have helped hundreds of thousands of people around the world who are drug dependent, and they have helped reduce the spread of HIV,” says Dr. Woody. “The new injectable and implantable naltrexone formulations are really the new kids on the block. But they’re offering us more options in an area where we really need a lot of help.”
Treating Heroin Addiction in Russia
“Russia’s drug enforcement administration officials have a strong influence on drug policy, and they don’t believe that treatments that include drugs like methadone and buprenorphine are effective,” says Dr. Krupitsky. Government officials also fear that the medications will be misused and diverted to black markets, fueling further drug abuse and crime.
Therefore, most drug abuse treatment in Russia is delivered through a nationwide network of “narcology” hospitals. These hospitals offer 7 to 10 days of inpatient detoxification, including limited medications to help with the side effects of withdrawal, followed by 2 to 4 weeks of inpatient counseling.
Despite its investment in more than 25,000 inpatient beds for detoxification treatment, Russia continues to have one of the highest rates of heroin use in the world. About 30 to 40 percent of heroin users in Russia are also infected with HIV, and more than 90 percent with hepatitis C.
“Working with investigators in Russia has been a great way to apply lessons I’ve learned through clinical trial networks in the United States to other situations where there’s a great opportunity to improve public health,” says Dr. Woody. “We share data electronically, teleconference with Skype, and email article drafts back and forth. Technology makes international collaboration much easier.” He notes that the logistics of overseas clinical research—including institutional review board approvals—are similar to those of multisite clinical trials in the United States.
This study was supported by NIH grants DA017317, DA17009 and KO5 DA-17009.
Sources:
Krupitsky, E.; Zvartau, E.; Blokhina, E.; Verbitskaya, E.; Wahlgren, V.; Tsoy-Podosenin, M.; Bushara, N.; Burakov, A.; Masalov, D.; Romanova, T.; Tyurina, A.; Palatkin, V.; Slavina, T.; Pecoraro, A.; Woody, G. E. Randomized trial of long-acting naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Archives of General Psychiatry 69(9):973–981, 2012. Full Text
Krupitsky, E.; Nunes, E.V.; Ling, W.; Gastfriend, D.R.; Memisoglu, A.; Silverman, B.L. Injectable extended-release naltrexone (XR-NTX) for opioid dependence: long-term safety and effectiveness. Addiction 108(9):1628–37. 2013. Abstract
