A trial that compared buprenorphine/naloxone (Bup/Nx) to methadone produced no evidence that either medication damages the liver. Researchers concluded that Bup/Nx and methadone are equally safe for the liver, and Bup/Nx may be considered a first line alternative to the more established medication for treating opioid addiction.
Dr. Andrew Saxon at the Veterans Affairs Puget Sound Health Care System in Seattle, and Dr. Walter Ling at the University of California, Los Angeles Integrated Substance Abuse Program, conducted the trial with colleagues in the NIDA Clinical Trials Network. Dr. Saxon’s team randomly assigned 1,269 new patients in 8 U.S. opioid treatment programs to therapy with either Bup/Nx or methadone. The study findings reflect the experiences of 731 patients who provided blood samples for liver function tests at baseline, completed the 24 weeks of active treatment, and submitted blood for at least 4 of 8 scheduled tests of liver function during treatment. These tests include measuring the levels of two enzymes (alanine aminotransferase and aspartate aminotransferase) that the liver releases when it is injured.
Most trial participants maintained enzyme levels that indicate healthy liver function throughout the study (see Figure). In 15.5 percent, enzyme levels increased to higher than twice the upper end of the normal range, indicating some ongoing liver injury. A few patients developed extreme elevations to 10 times the upper limit of normal or had other laboratory signs of severe liver injury.
The percentages of Bup/Nx and methadone patients who experienced each outcome were so close as to be statistically equivalent, warranting the conclusion that both medications were similarly safe. Although the researchers could not definitively rule out the possibility that the medications contributed to some of the observed worsening of liver function, their analysis produced no evidence to this effect. Instead, they say the changes most likely resulted from hepatitis, the toxicity of illicit drugs, and impurities in those drugs. Infection with hepatitis B or C doubled a patient’s odds of a significant change in enzyme levels and was the only predictor of worsening liver function. Most extreme increases in enzyme levels occurred when a patient seroconverted to hepatitis B or C, or used illicit drugs during the study.
The researchers note that about 44 percent of those screened for the study did not meet its enrollment criteria, suggesting that the participant group was healthier than many who visit clinics for addiction treatment. The ineligible population was also older, had a higher rate of stimulant use, and was less likely to be white than patients in the enrolled group, suggesting that the evaluable patient group might not be representative of all opioid-dependent patient groups.
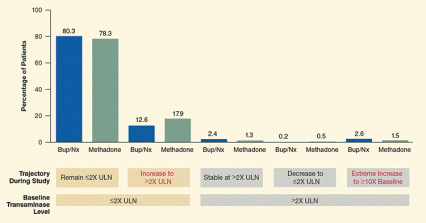 Figure. Researchers See No Evidence for Buprenorphine/Naloxone or Methadone Liver Damage The percentages of trial participants who incurred clinically significant transaminase increases during the study were similar among patients receiving buprenorphine/naloxone or methadone. Most of these increases occurred in conjunction with seroconversion to hepatitis B or C or injection drug use, and were not attributed to treatment medications.
Figure. Researchers See No Evidence for Buprenorphine/Naloxone or Methadone Liver Damage The percentages of trial participants who incurred clinically significant transaminase increases during the study were similar among patients receiving buprenorphine/naloxone or methadone. Most of these increases occurred in conjunction with seroconversion to hepatitis B or C or injection drug use, and were not attributed to treatment medications.About 90 percent of patients had baseline levels of transaminases (alanine transaminase and aspartate transaminase) below twice the upper limit of normal (2X ULN), which indicates healthy liver function. Most of these patients’ levels remained in the healthy zone throughout 24 weeks of opioid agonist treatment, but some patients’ levels rose above 2X ULN, indicating that they had developed clinically significant liver damage.
The remaining trial participants began the study with baseline transaminase levels above 2X ULN. Of these, some experienced a drop, some maintained stable, and some incurred a 10-fold increase above baseline of those levels, indicating significant deterioration of their already compromised liver function.
- Text description
-
The y-axis in this figure indicates the percentage of patients with certain liver transaminase levels. The x-axis shows which of two treatments (methadone or buprenorphine/naloxone) these patients underwent to treat their drug dependence. The x-axis also shows which patients at the beginning of the study showed levels of liver transaminases that were below twice the limit of normal levels and which patients showed transaminase levels above twice the limit of normal levels (indicating some harm to the liver), and how the transaminase levels changed over the course of the study (that is, whether for each of these two groups they stayed the same, dropped, or rose at one point during the study). Any differences between the effects of methadone and buprenorphine/naloxone treatments on liver health were statistically nonsignificant, indicating that neither drug was more harmful than the other. About 90 percent of the trial participants began the study with transaminase levels below twice the upper limit of normal, indicating a healthy liver. Most of those patients’ levels remained in the healthy zone, but some patients’ levels rose above twice the upper limit of normal, indicating that they had developed clinically significant liver damage. The other trial participants began the study with transaminase levels above twice the upper limit of normal. Of these, some experienced a drop in these levels, some maintained stable levels, and some experienced a 10-fold increase above baseline of those levels, indicating significant deterioration of their already compromised liver function.
This study was supported by NIH grants DA01714, DA013036, DA013045, DA015815, DA13038, DA13043, and DA013046.
Source:
Saxon, A.J.; Ling, W.; et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug Alcohol Depend. 128(1–2):71–76, 2013. doi: 10.1016/j.drugalcdep.2012.08.002. Epub 2012 Aug 22. Abstract
