Ketoprofen, an anti-inflammatory agent commonly prescribed to treat arthritis, reduces neuronal damage in rats that have been exposed to chronic stress and methamphetamine. If this finding of a recent NIDA-supported study extrapolates to humans, anti-inflammatory medications may gain a place in the treatment of methamphetamine addiction.
Chronic exposure to methamphetamine damages dopamine and serotonin terminals on neurons in the striatum, reducing levels of these neurotransmitters and impairing communication between neurons in this brain area. Past studies have shown that stress worsens the damage.
Drs. Nicole Northrop and Bryan Yamamoto, of the University of Toledo, Ohio, gave animals ketoprofen to investigate the role of inflammation in producing these effects, which contribute to methamphetamine users’ cognitive and behavioral problems. Their results indicate that neurotransmitter depletion in animals exposed to both methamphetamine and stress has two components, related to the two exposures. They implicate ketoprofen’s main target, the pro-inflammatory enzyme cyclooxygenase (COX-1/COX-2), in the stress-related component, and they indicate that a different mechanism underlies the methamphetamine-related component.
Partial Rescue
Drs. Northrop and Yamamoto have been conducting broad research on the impact of inflammation on brain health. They, along with others, have shown that stress increases COX-2 in the brain and that COX-2 activity promotes inflammation that can be toxic to brain cells. In the present study, they hypothesized that these effects are the link between stress and neuronal damage in methamphetamine abusers.
Ketoprofen provided the researchers with a tool to test their hypothesis. The medication inhibits COX-1/COX-2. If the hypothesis were correct, the medication should alleviate the stress-induced inflammatory response and resulting exacerbation of neuronal damage.
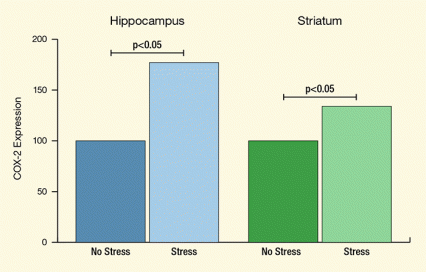 Figure 1. Chronic Stress Elevates Levels of Neuroinflammatory COX-2 Protein in the Rat Brain Rats were stressed or left unstressed for 10 days, and levels of the COX-2 protein, a neuroinflammatory mediator, were measured in the hippocampus (blue bars) and striatum (green bars) of these animals. Units for COX-2 expression on the y-axis represent percentages relative to the “no stress” treatments. In the stressed animals, the amount of COX-2 was increased by almost 80 percent in the hippocampus and by about 30 percent in the striatum relative to the levels in these brain regions in the unstressed animals.
Figure 1. Chronic Stress Elevates Levels of Neuroinflammatory COX-2 Protein in the Rat Brain Rats were stressed or left unstressed for 10 days, and levels of the COX-2 protein, a neuroinflammatory mediator, were measured in the hippocampus (blue bars) and striatum (green bars) of these animals. Units for COX-2 expression on the y-axis represent percentages relative to the “no stress” treatments. In the stressed animals, the amount of COX-2 was increased by almost 80 percent in the hippocampus and by about 30 percent in the striatum relative to the levels in these brain regions in the unstressed animals.
- Text description of Figure 1
-
The y-axis of the graph in this figure shows levels of expression of the enzyme COX-2, a neuroinflammatory protein, in the rat brain; the units for COX-2 expression on this axis represent percentages relative to the “no stress” treatments (indicated on the x-axis). The x-axis shows COX-2 enzyme expression for two brain regions, the hippocampus (indicated by blue bars) and the striatum (indicated by green bars) and after stress (light-colored bars) or no stress (dark-colored bars). The graph shows that the stressed rats had COX-2 levels that were increased by almost 80 percent in the hippocampus and by about 30 percent in the striatum relative to the levels in these brain regions in the “no stress” animals—the horizontal lines above the bars indicate the statistical comparisons and significance of these differences (denoted by “.05” above the lines, indicating a probability value of less than 0.05).
The researchers exposed rats to chronic stress, methamphetamine, or chronic stress followed by methamphetamine. To put rats into a condition of chronic stress, the researchers subjected them to a 10-day regimen of randomly timed cage agitation, food and water deprivation, cold, and isolation. This regimen increased COX-2 in the striatum by 32 percent (Figure 1).
Ketoprofen did not affect neurotransmitter levels among rats that were exposed to chronic stress alone or methamphetamine alone. However, it prevented the incremental damage produced by chronic stress among the animals that underwent both the stress regimen and methamphetamine exposure (see Figure 2). Without ketoprofen, chronic stress added a further 49 percent and 35 percent, respectively, to the striatal dopamine and serotonin depletions observed among animals that received methamphetamine alone. With ketoprofen, there was no significant difference.
The anti-inflammatory medication worked equally well whether the researchers gave it to the animals during the stress regimen or during methamphetamine administration. Hence, the anti-inflammatory treatment alleviated the adverse impact of stress even when it was administered after the stressful experience.
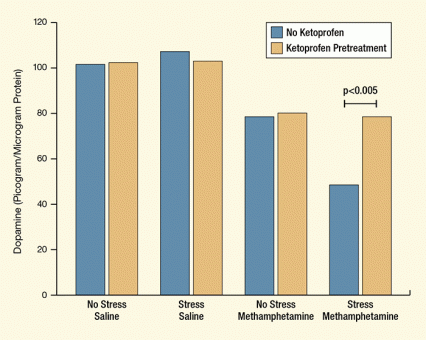 Figure 2. Ketoprofen Alleviates Stress-Related Exacerbations of Methamphetamine-Induced Dopamine Depletions in the Striatum Stress exacerbated methamphetamine-induced depletion of dopamine levels in the striatum of the rat brain, and ketoprofen canceled the exacerbation. Rats were subjected to stress or handled normally (“no stress” controls) and then injected 4 times with 7.5 mg/kg methamphetamine or saline every 2 hours; rats treated with ketoprofen were injected with 5 mg/kg of the drug 1 hour before each methamphetamine or saline injection.
Figure 2. Ketoprofen Alleviates Stress-Related Exacerbations of Methamphetamine-Induced Dopamine Depletions in the Striatum Stress exacerbated methamphetamine-induced depletion of dopamine levels in the striatum of the rat brain, and ketoprofen canceled the exacerbation. Rats were subjected to stress or handled normally (“no stress” controls) and then injected 4 times with 7.5 mg/kg methamphetamine or saline every 2 hours; rats treated with ketoprofen were injected with 5 mg/kg of the drug 1 hour before each methamphetamine or saline injection.
- Text description of Figure 2
-
The y-axis of the graph in this figure shows the concentration of the neurotransmitter dopamine (in picogram per microgram of protein) in the rat striatum. The x-axis shows four different experimental treatments the rats underwent: no stress and saline, stress and saline, no stress and methamphetamine, and stress and methamphetamine. For each treatment, the graph shows the results of pretreatment of the animals with ketoprofen (light orange bars) and without ketoprofen pretreatment (blue bars). The graph shows that the ketoprofen pretreatment increased dopamine levels only in the stress and methamphetamine treatment (indicated by the right-most pair of bars)—the horizontal lines above the two bars in this treatment indicate the statistical comparison and significance of this difference (denoted by “p<0.005” above the lines, indicating a probability value of less than 0.005).
A Double-Whammy for the Brain
The precise mechanism whereby COX-2 activation promotes neuronal damage remains unclear. The Ohio researchers tested and disproved one hypothesis. Based on the observation that COX-2 enhances prostaglandin production, they posited that the resulting increased prostaglandin activity might set off toxic reactions at neuronal EP1 prostaglandin receptors. However, when they administered an agent that inactivates the EP1 receptor, stress still aggravated the methamphetamine-related neurotransmitter depletion.
“There must be some other role for COX-2 activity to produce neuroinflammation,” says Dr. Yamamoto.
His current leading hypothesis draws on two well-established observations: Methamphetamine acutely increases dopamine levels in the striatum, and COX-2 converts dopamine into quinones, highly reactive chemicals that damage neurons. Hence, stress-related increases in COX-2 might combine with methamphetamine-related elevations in dopamine to produce a bumper crop of destructive quinones.
Dr. Yamamoto says that this scenario is consistent with another finding from the study: Although ketoprofen protected neurotransmitter levels in the striatum, it did not do so in the hippocampus, even though stress increased hippocampal COX-2 by 77 percent. Compared to the striatum, dopamine levels are low in the hippocampus, providing little substrate for COX-2 to convert into quinones.
Treating Drug-Induced Neuroinflammation
Drs. Yamamoto and Northrop and colleagues have previously shown that the combination of stress and methamphetamine compromises the integrity of the blood–brain barrier—the protective cell layer that lines the capillaries in the brain and prevents large molecules and pathogens, including bacteria and viruses, from entering the brain. In those studies, ketoprofen alleviated long-term blood‒brain barrier disruption caused by the combination of methamphetamine and stress, but not by methamphetamine alone.
The parallel findings of these studies raise the possibility that treatment with ketoprofen or other anti-inflammatory agents might alleviate some of methamphetamine users’ neuronal damage and resulting problems. Ketoprofen has been clinically approved for human use, so if further research supports the use of anti-inflammatory drugs to mitigate the neurotoxicity of methamphetamine, human trials could begin promptly.
For the present, however, Dr. Yamamoto concurs with Dr. Jerry Frankenheim of NIDA’s Functional Neuroscience Research Branch, who cautions, “There is not yet enough evidence to warrant the use of anti-inflammatory drugs to protect against inflammation resulting from methamphetamine and stress.” Dr. Frankenheim adds, “The Toledo team’s preclinical study was cleverly designed to identify the mechanisms by which stress increases the neurotoxicity of methamphetamine, but not to identify a treatment.” Moreover, he notes that ketoprofen did not protect the hippocampus, and ketoprofen has side effects, including gastrointestinal bleeding.
Implications Beyond Drug Abuse
The finding that neuroinflammation can damage dopamine and serotonin neurons may be important for many neurodegenerative disorders. “Even though this study focused on methamphetamine, a lot of the underlying mechanisms we’re investigating are similar to those thought to be at work in neurodegenerative diseases in which inflammation has been implicated, such as Parkinson’s and Alzheimer’s,” says Dr. Yamamoto. “Perhaps injuries produced by drugs of abuse may not be much different from damage exhibited by these disease conditions.”
In addition, Dr. Yamamoto says, researchers need to consider how inflammation outside the brain might contribute to inflammation in the brain. “We’ve always considered the brain in isolation—that drugs act directly in the brain to produce these effects,” he says. “But the cross talk between the brain and the rest of the body also needs to be investigated further.” Dr. Yamamoto points to a previous research finding that liver damage contributes to methamphetamine-induced brain damage.
Dr. Frankenheim agrees: “Neuroinflammation is a frontier research area right now,” he says. “From many studies of neurodegenerative and psychiatric disorders, a common theme is emerging: Neuroinflammation damages the brain, and more experiments along the lines of this work should be done.”
This study was supported by NIH grant DA007606.
Sources:
Northrop, N.A. and Yamamoto B.K. Cyclooxygenase activity contributes to the monoaminergic damage caused by serial exposure to stress and methamphetamine. Neuropharmacology 72:96–105, 2013. Abstract
Northrop, N.A. and Yamamoto B.K. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. Journal of Neuroimmune Pharmacology 7(4):951–968, 2012. Full text
