Microneedles have notched a significant advance on the way to clinical usefulness: A single treatment using the transdermal technique delivered therapeutic doses of the opioid antagonist naltrexone continuously for 7 days.
Microneedles are an innovative technique for delivering medications through the skin, a route that could particularly benefit patients receiving naltrexone therapy for opioid and alcohol dependence. Both current methods of administering naltrexone have drawbacks. The oral formulation is short-acting, leading to problems with compliance; it can be difficult to dose optimally because of individual differences in metabolism; and it can cause liver damage. The injectable formulation requires monthly visits to a physician for a potentially painful intramuscular shot. In contrast, transdermal medication delivery has potential to provide convenient and painless stable dosing over extended periods while reducing hepatic exposure.
The microneedles system is designed to surmount a technical issue that to date has thwarted realization of the clinical promise of transdermal naltrexone: In contrast to other medications that are administered via conventional skin patches, naltrexone does not pass through intact skin. Microneedles treatment breaches this barrier: The clinician presses a dime-sized array of microneedles briefly into the patient’s skin, opening a grid of micropores. The microneedles are withdrawn, and a medication-impregnated patch is applied over the grid. The micropores diffuse the medication through the epidermis into the microcirculation and bloodstream.
Microneedles worked as intended in an initial proof-of-concept study (see Skin Patch Proves Effectiveness of New Technology). However, the researchers observed that the skin’s natural healing process begins to close off the micropores after 2 or 3 days. In clinical practice, this would create a need for frequent re-treatment that would limit the system’s practicality and potential acceptability to patients.
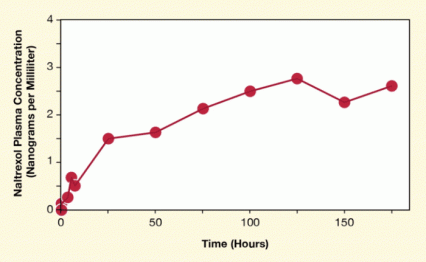 Figure. Microneedles Enable Continuous Transdermal Drug Delivery for 7 Days With diclofenac to keep micropores patent, a single microneedles application sustained therapeutic concentrations of naltrexone for 1 week. The graph shows levels of naltrexol, the active metabolite of naltrexone, in blood plasma.
Figure. Microneedles Enable Continuous Transdermal Drug Delivery for 7 Days With diclofenac to keep micropores patent, a single microneedles application sustained therapeutic concentrations of naltrexone for 1 week. The graph shows levels of naltrexol, the active metabolite of naltrexone, in blood plasma.
- Text description of Figure
-
The figure shows a line graph indicating the concentration of naltrexol—a product of the biochemical conversion of naltrexone—in blood plasma over a period of 7 days (168 hours). The vertical (y)-axis shows the concentration of naltrexol in plasma (in nanograms per milliliter). The horizontal (x)-axis shows the time in hours during which naltrexol was measured. The measurements were performed with plasma from people wearing a naltrexone microneedle patch. As shown by the data symbols (red circles) connected by the red line, levels of naltrexol increased at early time points (in the first 25 hours after the start of the naltrexone microneedle treatment) and remained high until the end of the 7-day treatment.
In the new study, Dr. Nicole K. Brogden a predoctoral student at the University of Kentucky, and faculty member Dr. Audra L. Stinchcomb, demonstrated in principle that the micropore closure problem can be overcome. (Dr. Brogden is now an Associate on the faculty of the University of Iowa, and Dr. Stinchcomb continues to develop microneedles and other transdermal medication systems as chief scientific officer and CEO at AllTranz Inc.). In a trial with a group of healthy volunteers, rubbing a topical gel preparation of the anti-inflammatory medication diclofenac onto the micropore site before applying the microneedles—and every 2 days thereafter—kept the pores open and the medication flowing smoothly for 7 full days (see Figure).
Although diclofenac’s anti-inflammatory activity seems to play a role in preventing micropore closure, the exact mechanisms are unclear. The researchers suggest that diclofenac’s antiproliferative effects on skin restoration processes may need to be examined to further understand the process.
A milestone has been reached, but more work is needed. The researchers say that diclofenac may turn out not to be the best formulation for maintaining micropore patency. Trial participants required multiple medication patches to sustain therapeutic dosing, possibly because a chemical interaction between the two compounds decreased naltrexone absorption through the micropores. The researchers are now seeking more efficient alternatives.
This study was supported by NIH grants F31DA029374, 1RR033173-01, R01DA013425, and R42DA032191.
Source:
Brogden, N.K.; Banks, S.L.; Crofford, L.J.; Stinchcomb, A.L. Diclofenac enables unprecedented week-long microneedle-enhanced delivery of a skin impermeable medication in humans. Pharmaceutical Research 30(8):1947-1955, 2013. Abstract
