Marijuana use makes tobacco use more pleasurable and may increase the user’s risk for becoming addicted to nicotine. These are the implications of recent experiments in which exposing rats to delta-9-tetrahydrocannabinol (THC) caused the animals to self-administer nicotine more readily and work harder for it.
Most marijuana users smoke cigarettes, and about 1 in 5 individuals who use both substances (1 in 3 among African Americans) used marijuana first. In one recent study, adolescents who used marijuana weekly were more likely than less frequent marijuana users or nonusers to initiate tobacco use. These patterns occur in part because some of the same personal traits and social and environmental exposures that lead people to use marijuana also influence them to try other drugs. The new findings suggest that marijuana use itself, independently of these influences, predisposes users to become regular smokers, increasing their odds of becoming addicted to nicotine.
THC Primes Nicotine Self-Administration
Dr. Leigh Panlilio and Dr. Steven Goldberg and colleagues at NIDA’s Intramural Research Program (IRP), the University of Texas Health Science Center at San Antonio, and the University of Poitiers in France tested the hypothesis that THC, the psychoactive ingredient in marijuana, might be a “gateway” drug for nicotine. The hypothesis posits that THC pharmacologically sensitizes the brain to be more responsive to nicotine’s rewarding and addictive effects, so that marijuana users who try tobacco are more likely to continue using it.
To isolate the pharmacological effect of THC from other potential influences on nicotine consumption, the researchers conducted their experiment with laboratory rats. The animals were genetically nearly identical and not subject to the many social and environmental differences that affect whether or not, or how much, a person uses nicotine.
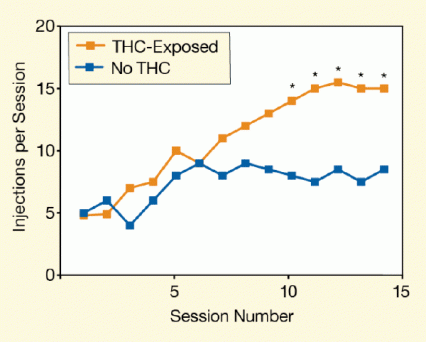 Figure 1. Rats Pre-Exposed to THC Self-Administer Nicotine at Higher Rates Than Unexposed Rats Beginning with the 10th session of nicotine self-administration, THC-exposed rats self-administered significantly more nicotine injections than animals that had not been exposed to THC. The asterisks indicate statistically significant (p <.02) differences between THC-exposed and unexposed animals.
Figure 1. Rats Pre-Exposed to THC Self-Administer Nicotine at Higher Rates Than Unexposed Rats Beginning with the 10th session of nicotine self-administration, THC-exposed rats self-administered significantly more nicotine injections than animals that had not been exposed to THC. The asterisks indicate statistically significant (p <.02) differences between THC-exposed and unexposed animals.
- Text description of Figure 1
-
The figure shows a line graph indicating the number of times rats requested and received nicotine injections during experimental sessions that took place after the animals had been pre-exposed to THC or not been exposed to THC (control rats). The vertical (y)-axis shows the number of injections per session and the horizontal (x)-axis the session numbers in numerical order. Rats that had been pre-exposed to THC (orange line and symbols) consistently requested and received a greater number of nicotine injections at and after the 10th session than the control rats (blue line and symbols). In these later sessions, the pre-exposed rats received on average 15 injections per session, whereas the unexposed rats always received less than 10 injections per session. Asterisks above the symbols for THC–pre-exposed rats for sessions 10 and higher indicate that the number of injections per session were statistically significantly different from those of the control rats (p <.02).
The researchers injected one group of rats with THC and another with saline solution on 3 consecutive days. Starting 1 week after the final THC injection, they gave the animals the opportunity to self-administer nicotine during 2-hour daily test sessions for 14 days. To obtain a nicotine infusion, an animal had to poke its nose into a hole in the wall of its testing chamber. Among the 17 rats pre-exposed to THC, 16 (94 percent) established consumption behavior, the criterion for which was self-administration of 10 or more nicotine doses (30 micrograms per dose) in 3 daily sessions in a row. Among the 17 saline-treated controls, only 11 (65 percent) met this criterion. Moreover, of the rats that met the criterion, those exposed to THC self-administered a higher cumulative amount of nicotine than those in the saline-exposed group (see Figure 1).
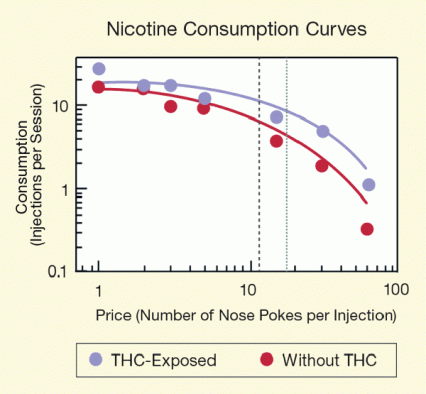 Figure 2. THC Exposure Increases Rats’ Motivation To Obtain Nicotine On average, THC-exposed animals were motivated to obtain a nicotine injection when researchers raised the price to 17 nose pokes. Saline-exposed control animals’ motivation dropped off when the price rose above 11 nose pokes.
Figure 2. THC Exposure Increases Rats’ Motivation To Obtain Nicotine On average, THC-exposed animals were motivated to obtain a nicotine injection when researchers raised the price to 17 nose pokes. Saline-exposed control animals’ motivation dropped off when the price rose above 11 nose pokes.
- Text description of Figure 2
-
The figure shows demand for and consumption of nicotine of rats pre-exposed to THC and rats that had not been exposed to THC (controls) in response to a change in price for receiving nicotine. The vertical (y)-axis shows the number of nicotine injections the animals received per session, and the horizontal (x)-axis the price for receiving an injection, measured as the number of nose pokes a rat had to execute before receiving a nicotine injection. Rats pre-exposed to THC (purple line and dots) consistently demanded and received a higher number of nicotine injections than the control rats (red line and dots). The rats that had been pre-exposed to THC on average showed a maximal response rate of 17.1 nose pokes per nicotine injection, whereas the control rats gave an average maximal response rate of 11.4 nose pokes per injection, indicating that THC exposure significantly increased the price the animals were willing to pay to receive nicotine.
The THC-treated rats also invested more energy in seeking nicotine, indicating that THC exposure increased motivation to get the drug. To achieve this observation, the researchers ratcheted up the number of times the rats needed to nose-poke for a nicotine dose. On average, the THC-exposed animals’ motivation still remained high when up to 17 pokes were required to obtain each dose. In contrast, the saline-exposed animals’ motivation dropped off when more than 11 pokes were required (see Figure 2).
Dr. Goldberg calls attention to the relatively long time interval between THC exposure and nicotine consumption in the study. “It is striking that the animals went a whole week without receiving any THC before we let them self-administer nicotine, yet they were still working harder for nicotine at the end of the experiment, more than a month after their last THC exposure. These observations demonstrate that the THC effect is persistent,” says Dr. Goldberg. He and his colleagues suggest that this persistence indicates that THC causes lasting changes to the brain.
THC and Signaling Systems
While the mechanisms whereby THC might sensitize the brain to nicotine remain to be worked out, Dr. Panlilio and colleagues stress that the brain signaling systems activated by THC and nicotine overlap. Moreover, some studies have suggested that cannabinoid receptors—the sites on neurons where THC binds and initiates its pharmacological effects—play a role in nicotine addiction. In fact, chemical blockade of cannabinoid receptors has been shown to weaken the reinforcing effect of nicotine sufficiently to be considered a potential strategy for treating nicotine addiction.
THC also has been shown to produce lasting changes in the opioid signaling system, which is thought to contribute to nicotine reward, tolerance, and withdrawal. THC alters both cannabinoid and opioid signaling in the nucleus accumbens, a brain region that is implicated in addiction. Accordingly, the researchers suggest that THC might promote nicotine consumption via an interaction between the brain’s cannabinoid, opioid, and nicotinic acetylcholine systems.
No Effect on Heroin or Cocaine
THC’s priming effect appears to be specific to nicotine. In other experiments, Dr. Panlilio and colleagues found that exposing animals to THC did not result in increased self-administration of cocaine or heroin: For both of those drugs, equal proportions (80 percent) of both THC-pretreated and saline-pretreated animals met the 10-doses-per-day-for-3-consecutive-days criterion for established drug use. Nor were animals treated with THC willing to work harder for either of those drugs, compared with animals treated with saline. In fact, animals were less motivated to work for cocaine after being exposed to THC.
Studies have shown that people will go to great lengths to consume nicotine, and many who smoke try to quit but fail. Given the new evidence that exposure to THC intensifies nicotine’s rewarding and addictive effects in rats, the researchers recommend examining the possibility that marijuana makes people more prone to nicotine use and addiction.
Sources:
Panlilio, L.V.; Zanettini, C.; Barnes, C.; Solinas, M.; Goldberg, S.R. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology 38(7):1190-1208, 2013. Abstract
Panlilio, L.V.; Solinas, M.; Matthews, S.A.; Goldberg, S.R. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology 32(3):646-657, 2007. Abstract
