Recent observations in animals may help explain why reminders of past cocaine use exert uniquely powerful influence over the behavior of people with addiction. NIDA-supported researchers identified a brain response that occurs when rats encounter a cue that they associate with previous cocaine self-administration, but not a cue associated with a pleasurable non-drug experience. Moreover, the response correlates in time and intensity with the animals’ cue-induced relapse to cocaine-seeking.
The nature of the response is such that it might be experienced as a powerful urge to obtain the drug, one that forecloses on the ability to compare alternative courses of action. The response takes place in the nucleus accumbens (NAc), a brain region that receives signals from the brain’s motivational and decision-making circuits and relays them to the motor circuits. Called synaptic potentiation (SP), the response makes NAc neurons more sensitive to incoming excitatory signals. This greater sensitivity alters the NAc output to the brain’s motor circuits in a way that promotes initiation of behaviors such as relapsing to a drug of abuse.
Dr. Peter Kalivas, Dr. Cassandra Gipson, and colleagues at the Medical University of South Carolina (MUSC) in Charleston conducted the experiments. Their findings suggest that treatments to prevent or diminish SP in the NAc might help prevent people with cocaine addiction from relapsing.
What’s Controlling Hard-To-Control Urges?
A drug-associated cue can be anything that a person with a substance use disorder associates with their previous drug use—the tactile feel of a pack of cigarettes, the sights or smells of a place where they have used drugs, or even a conversation that triggers a memory of drug use. “These cues become woven into the fabric of people’s lives, making it hard to avoid them and increasing the chance of relapse,” explains Dr. Kalivas.
The MUSC team hypothesized that cocaine-associated cues would trigger SP in the synapses of medium spiny neurons in the core of the NAc of rats, and that this would promote the reinstatement of relapse-like behavior. To test this hypothesis, they exposed rats to a three-stage protocol that mimics human cocaine use and the association of such use with a cue; withdrawal; and relapse:
- Use and association. Rats self-administered cocaine in a chamber with a lever that, when pressed, delivered an infusion of the drug accompanied by a tone-and-light cue.
- Withdrawal. After the rats had self-administered cocaine for 10 days, the researchers adjusted the apparatus so that pressing the lever no longer delivered the drug or the cue. The rats, their motivation no longer reinforced by the drug, tapered off pressing the lever.
- Relapse. After the rats had been in the withdrawal stage for 2 weeks, the researchers adjusted the apparatus again, this time so that pressing the lever produced only the tone-and-light cue. The rats reverted to avid lever pressing in response to the cue, even though the lever still did not deliver cocaine.
The cocaine-exposed rats’ reinstated lever pressing resembles the compulsive behavior of people with addiction when they relapse after encountering a drug cue. To understand the brain mechanisms underlying this behavior, the researchers examined NAc tissue samples taken from the experimental animals at intervals throughout the 2-hour cue-reinstatement session.
Potentiation and Lever Pressing
The tissue samples revealed that synapses in the NAc of the cocaine-exposed animals underwent two changes in response to the cocaine-associated cues:
- An increase in the strength of electrical currents transmitted through one of the two main types of glutamate receptor (AMPA; α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) in the synapse (see Figure 1)
- Growth in the size of the dendritic spine heads on medium spiny neurons, which are the main type of neurons found in the NAc (see Figure 2)
Both of these changes reflect SP: Increased AMPA current directly indicates greater cell sensitivity to glutamate stimulates the cell via those receptors. Increased dendritic spine head size suggests that there is a larger population of receptors available for glutamate to stimulate.
“The spine head enlargement in the cocaine-exposed rats is very transient, which corresponds with the rats’ behavioral output,” says Dr. Gipson. She points out that the increases in AMPA current and spine head size were both tightly linked to the animals’ relapse-like behavior. They paralleled the trajectory of the animals’ lever-pressing frequency—rising to a peak 10 to 15 minutes after the animals encountered the restored cue, then tailing off over the next couple of hours as the animals learned that the cocaine experience was not forthcoming.
SP in the NAc appeared to be a pathology specifically related to drug cues, rather than a general response to cues related to desirable substances. To establish this, the researchers exposed a separate set of rats to the same three-stage experimental protocol, but with sucrose as the motivational reinforcer instead of cocaine. Although these rats reinstated lever pressing in the relapse stage of the protocol, the neurons in their NAc developed neither the rapid, transient growth in dendritic spine head nor the changes in AMPA current.
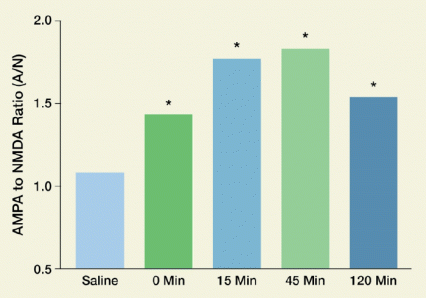 Figure 1. Excitatory Currents in Neurons of the Nucleus Accumbens Core Increase Within 15 Minutes of Cue-Induced Cocaine Relapses The current induced by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor relative to that induced by the N-methyl-D-aspartate (NMDA) receptor indicates the level of excitation in neurons. Compared with control animals receiving saline, cocaine-exposed rats had significantly increased AMPA-to-NMDA ratios in their nucleus accumbens core neurons before a cue-induced relapse (T = 0, green bar), indicating synaptic potentiation. The ratio increased for another 15 minutes after the relapse (T = 15, blue bar); stayed at the same level until 45 minutes (T = 45, light-green bar); and after 120 minutes (T = 120, dark-blue bar) declined to levels similar to those before the relapse. Asterisks indicate that the ratios in the cocaine-exposed animals were statistically significantly different from those in the control animals (p<0.05).
Figure 1. Excitatory Currents in Neurons of the Nucleus Accumbens Core Increase Within 15 Minutes of Cue-Induced Cocaine Relapses The current induced by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor relative to that induced by the N-methyl-D-aspartate (NMDA) receptor indicates the level of excitation in neurons. Compared with control animals receiving saline, cocaine-exposed rats had significantly increased AMPA-to-NMDA ratios in their nucleus accumbens core neurons before a cue-induced relapse (T = 0, green bar), indicating synaptic potentiation. The ratio increased for another 15 minutes after the relapse (T = 15, blue bar); stayed at the same level until 45 minutes (T = 45, light-green bar); and after 120 minutes (T = 120, dark-blue bar) declined to levels similar to those before the relapse. Asterisks indicate that the ratios in the cocaine-exposed animals were statistically significantly different from those in the control animals (p<0.05).
- Text description of Figure 1
-
The figure shows a bar graph of excitatory currents in nerve cells of the nucleus accumbens before and after a cue-induced relapse in rats trained to associate the cue with the effects of cocaine or of an inactive control (saline). The vertical (y)-axis shows the ratio in the currents induced by two different neuron receptors: the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors. A higher AMPA-to-NMDA ratio means a higher level of excitation in the neurons. The horizontal axis shows the treatments and the time points after the relapse was induced in the animals. Those that had received the saline control had a statistically significantly lower AMPA-to-NMDA ratio than animals that had received cocaine (indicated by asterisks above the cocaine-exposed rats, denoting p<0.05 for this difference). After the cocaine-exposed animals received the relapse cue, the ratio increased between 0 minutes and 15 minutes, continued to rise until 45 minutes, and declined after 120 minutes to about the same size as before the relapse.
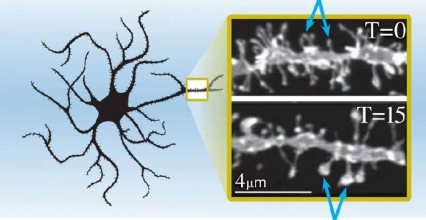 Figure 2. Spine Heads of Neurons in the Nucleus Accumbens Core Enlarge 15 Minutes After a Cue-Induced Cocaine Relapse As shown by the blue arrows, the spine heads of medium spiny neurons in the nucleus accumbens core of cocaine-trained rats at 15 minutes after a cue-induced relapse (T = 15) are larger than those in the same rats before the induced relapse (T = 0).
Figure 2. Spine Heads of Neurons in the Nucleus Accumbens Core Enlarge 15 Minutes After a Cue-Induced Cocaine Relapse As shown by the blue arrows, the spine heads of medium spiny neurons in the nucleus accumbens core of cocaine-trained rats at 15 minutes after a cue-induced relapse (T = 15) are larger than those in the same rats before the induced relapse (T = 0).
- Text description of Figure 2
-
The left side of the figure shows a schematic of medium spiny neurons of the nucleus accumbens core. A small area of the schematic—highlighted by the rectangle—provides an impression of the relative location and size of the spine heads shown in two photographs on the right. The two photographs show the neurons in the rats trained to associate a cue with cocaine. The upper image shows the spine heads of the neurons before the rats experienced a cue-induced relapse to cocaine-seeking and the lower image the spine heads 15 minutes after the induced relapse. Comparisons between the spine heads in the two images reveal that the spine heads became enlarged 15 minutes after the relapse relative to those before the relapse (highlighted by blue arrows pointing to selected spine heads in both panels).
A Question of Value
“This evidence strongly suggests that synaptic potentiation underlies the difficulty people have in controlling their drug-seeking behavior,” Dr. Kalivas says. “Once they experience the cues, their brains undergo supersensitivity to glutamate in the NAc, driving their drug-seeking.”
Why would SP in the NAc have that effect? Dr. Gipson and Dr. Kalivas explain that the NAc receives glutamate signals from the prefrontal cortex (PFC), a brain area that assigns importance to environmental cues of all sorts. When the PFC assigns high importance to a cue—as the PFC of an addicted person does to cocaine cues—it sends a strong glutamate signal to the NAc, indicating urgency to respond. SP of the synapses in the NAc amplifies the impact of these signals still further and relays them to the motor neurons, which initiate the cued behavior—in this case, drug-seeking.
Consistent with this account, Dr. Gipson repeated her experiment and showed that inhibiting the PFC from releasing glutamate to the NAc prevented rats’ relapse-like response to cocaine-associated cues. PFC inhibition, achieved by injecting a cocktail of inhibitory drugs into the rats’ prelimbic cortex (a region of the PFC), also prevented SP: These animals exhibited no spine head enlargement or increase in AMPA currents.
From Insight to Treatment
The MUSC group has been extensively investigating the role of glutamate in addiction and drug relapse. They and others have demonstrated that animals that have been withdrawn from self-administered cocaine exhibit long-lasting changes in glutamate signaling in the NAc. Their new findings support a conjecture that these changes may predispose animals to revert to drug-seeking when exposed to stimuli including stress, new drug exposures, and cocaine-associated cues.
Dr. Kalivas’ laboratory is currently testing a compound that reverses some of the alterations in glutamate signaling that cocaine addiction causes. “By targeting the glutamate system, one might short-circuit the spine head enlargement and synaptic potentiation, and therefore lower the compulsion to relapse,” he says.
The MUSC researchers have also demonstrated that heroin and nicotine cause synaptic strengthening in the NAc. “This effect extends to at least three drugs of abuse that are very different from each other,” he says. “That may mean that the neurobiology of the relapse event, a defining characteristic of addiction, is shared.” If so, a behavioral or pharmacological approach that targets SP might work for multiple types of substance use disorders.
The discovery of brain response that is particular to drug cues is potentially very significant, says Dr. Jerry Frankenheim of NIDA’s Functional Neuroscience Research Branch in the Division of Basic Neuroscience and Behavioral Research: “These findings provide encouragement that we may be able to block drug relapse without interfering with motivation that we need to get out of bed in the morning, go to work, eat, and otherwise enjoy life.”
This study was supported by NIH grants DA003906, DA007288, DA012513, DA015369, and DA033690.
Source:
Gipson, C.D.; Kupchik, Y.M.; Shen, H.; Reissner, K.J.; Thomas, C.A.; Kalivas, P.W. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron77(5):867-872, 2013. Full text
