Two recent studies represent early steps toward the goal of personalized therapy for pain and addiction based on patients’ genetic makeup. One study associated a rare variant of the gene for the μ-opioid receptor (OPRM1) with a decreased risk for addiction to heroin or cocaine. The other linked variants in two genes—OPRM1, and for the enzyme catechol-O-methyltransferase (COMT)—with reduced severity of neonatal abstinence syndrome (NAS) in newborns who were prenatally exposed to methadone or buprenorphine.
The μ-opioid receptors are relay stations for cell-to-cell signaling in the body’s natural opioid system. They are also the sites where opioid drugs influence that system. The drugs massively amplify signaling within the system and so can greatly enhance its beneficial functions—in particular, pain suppression. However, they also can cause severe adverse effects and addiction. The two recent studies suggest that genotyping may enable clinicians to base therapies on individual patients’ potential responsiveness to opioid drugs’ therapeutic effects and vulnerability to their harmful effects.
Rare and Valuable
Dr. Toni-Kim Clarke and researchers from the University of Pennsylvania Perelman School of Medicine and the Philadelphia VA Medical Center found that a particular variant (allele) within the OPRM1 gene was only half as prevalent among a cohort of European Americans who were addicted to heroin or cocaine as among a comparison cohort drawn from the general population. Since alleles that protect against drug addiction are expected to be less common among those using drugs than among nonusers, the researchers concluded that the allele protects against the risk for heroin or cocaine dependence.
The protective allele is a rare variant of the single-nucleotide polymorphism (SNP) rs62638690 (see Figure 1). It occurred in 0.38 percent of addicted individuals in the study and 0.79 percent of those in the comparison group. It consists of a change in a single unit of DNA (a thymine replaces guanine), which in turn alters the μ-opioid receptor by changing one of its component amino acids, cysteine, to another, phenylalanine. Other investigators have found that this change makes the receptor less responsive to some opioids, so that higher concentrations of the drugs are required to activate it. However, says Dr. Clarke, “It's not yet known whether this reduced potency is due to changes in binding to the receptor or to changes in the receptor’s location in the cell membrane. These are questions for further research.”
For their study, Dr. Clarke and colleagues analyzed DNA from 1,377 drug-addicted European Americans who had participated in previous NIDA-supported studies and contributed samples to a DNA research repository. The researchers compared the frequencies of the rs62638690 alleles in that group to the frequencies among 6,503 European Americans whose DNA had been previously analyzed and recorded in database that aims to represent the general population. Examination of three other SNPs within OPRM1 (rs17174794 among European Americans; rs17174801 and rs1799971 among African Americans) revealed no relationship to the risk for addiction.
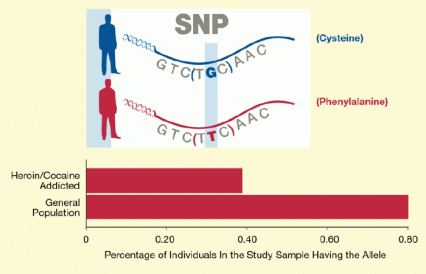 Figure 1. Among European Americans, the rare T allele of SNP rs62638690 Appears Protective against Drug Dependence The T allele was twice as frequent (0.79 percent to 0.38 percent) in a sample drawn from the general population compared to a group of patients addicted to heroin or cocaine. The allele occurs when thymine replaces guanine at a particular spot within the gene for the μ-opioid receptor. The substitution changes an amino acid in the receptor from cysteine to phenylalanine, which alters the receptor’s responses to opioids.
Figure 1. Among European Americans, the rare T allele of SNP rs62638690 Appears Protective against Drug Dependence The T allele was twice as frequent (0.79 percent to 0.38 percent) in a sample drawn from the general population compared to a group of patients addicted to heroin or cocaine. The allele occurs when thymine replaces guanine at a particular spot within the gene for the μ-opioid receptor. The substitution changes an amino acid in the receptor from cysteine to phenylalanine, which alters the receptor’s responses to opioids.
- Text description of Figure 1
-
The upper panel shows a schematic of a part of the OPRM1 gene sequence for the μ-opioid receptor containing a single nucleotide polymorphism (SNP), that is, a change from a guanine (“G”), shown in blue, to a thymine (“T”) shown in red. This SNP changes a specific amino acid in the protein sequence of the opioid receptor from a cysteine to a phenylalanine (shown to the right of the panel in colors matching those of the variant G and T nucleotides). The lower panel shows a bar graph indicating the proportion of people in the general population or addicted to heroin or cocaine who have the OPRM1 “T” allele causing the change to phenylalanine in the receptor. The vertical axis shows the two study populations (general and heroin/cocaine addicted), and the horizontal axis the percentage of people with the “T” allele. Almost 0.8 percent of the people in the general population had the “T” allele, twice as many as among those addicted to heroin or cocaine (about 0.4 percent), suggesting that this allele provides some protection against addiction to the two drugs.
OPRM1 and COMT Variants Attenuate NAS
Dr. Elisha M. Wachman at Tufts Medical Center and colleagues linked a variant of SNP rs1799971 in the OPRM1 gene to reduced severity of NAS. Among 86 newborns who were prenatally exposed to buprenorphine or methadone, 56 (65 percent) required morphine or methadone treatment to wean them off opioid dependence, and 21 (24 percent) were treated with additional medications to alleviate withdrawal symptoms. However, those infants with a guanine rs1799971 allele were, on average, about 25 percent less likely to require medical treatment for NAS than those with adenine alleles in both copies (see Figure 2). These infants, about 15 percent of those in the study, also were discharged from hospital care sooner: after 17 days, on average, compared to 24 days for the infants with only the adenine allele.
The study data also indicated that breast feeding reduced the severity of NAS and mothers’ cigarette smoking increased it. The researchers adjusted their analyses of the impact of the gene variant to allow for these effects and those of other factors known to affect NAS.
Dr. Wachman says, “We think that the newborns with the protective rs1799971 allele tolerate opioids better because the allele results in the production of fewer receptors to which the drugs can bind.” Previous studies have disclosed that the allele produces μ-opioid receptors that bind relatively strongly to one of the body’s natural opioids, β-endorphin. Because this binding reduces the number of receptors required to achieve adequate signaling strength within the brain’s opioid system, the brain produces fewer receptors. As a result, the fetal brain with the protective allele may be less affected by the drugs the mother takes during pregnancy. Accordingly, less morphine or methadone may be needed to manage neonatal withdrawal symptoms.
The researchers’ analyses also discovered associations between an allele of SNP rs4680 in the COMT gene, reduced need for medical treatment for NAS, and shorter length of hospital stay. The protective COMT allele conferred roughly the same degree of protection against NAS as the protective OPRM1 variant. The researchers suggest that the allele, which substitutes guanine for adenine, eases infants’ transition from opioid dependence by increasing their tolerance for stress.
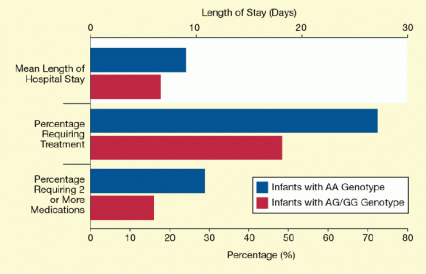 Figure 2. Newborns With the rs1799971 SNP in OPRM1 Had Shorter Hospital Stays and Were Less Likely to Require NAS Treatment Newborns carrying at least one copy of the rs1799971 rare variant (G) of the OPRM1 gene required shorter hospital stays and fewer medications for NAS treatment than those carrying only the common variant (A).
Figure 2. Newborns With the rs1799971 SNP in OPRM1 Had Shorter Hospital Stays and Were Less Likely to Require NAS Treatment Newborns carrying at least one copy of the rs1799971 rare variant (G) of the OPRM1 gene required shorter hospital stays and fewer medications for NAS treatment than those carrying only the common variant (A).
- Text description of Figure 2
-
The figure shows a bar graph indicating the effects of a SNP in the OPRM1 gene—i.e., a change from an adenine (“A”) to a guanine (“G”) at a specific position in the gene’s sequence—on NAS in newborns exposed to opioids in the womb. The upper horizontal axis shows the newborns’ length of stay in the hospital and the lower horizontal axis the percentages of newborns requiring treatment or two or more medications for NAS. As shown by the differences between the red and blue bars (indicating newborns with at least one “G” allele and those with only “A” alleles, respectively), newborns having at least one “G” allele in OPRM1 had on average shorter hospital stays than the newborns having only the “A” allele. In addition, fewer of the newborns with the “G” allele required treatment or two or more medications for NAS than among the newborns with only the “A” allele.
Genetic Markers for Individualized Treatments
Dr. Jamie Biswas, Chief of NIDA’s Medications Research Grants Branch, says that both studies highlight the importance of genetic factors in determining the outcomes of treatments for substance abuse: “The findings could help explain why some people respond well to treatment while others do not. They could provide a rationale in clinical trials of substance abuse treatments for dividing subjects into groups based on the subjects’ genetic variants.”
Dr. Jonathan M. Davis, lead investigator of the Tufts study, explains, “The incidence and severity of NAS symptoms vary widely among newborns and are not always related to the type and amount of drugs their mothers use.” If genetic variations can reliably predict whether an infant will have NAS, and its severity, then clinicians can use genetic testing to prepare appropriate treatment for opioid-exposed newborns. It might also inform adjustments in the treatment of expectant mothers’ opioid addiction to reduce the potential for severe NAS.
Dr. Biswas notes that the large population size in the study by Dr. Clarke and colleagues adds to the reliability of its findings, whereas the smaller size of the NAS study by Dr. Wachman makes its findings only preliminary. Even though it is too early to tell whether these findings can be applied to clinical trials and practice, “the study of the role of genetics in treating diseases is valid and highly promising. It is also being pursued for many other health and disease indications—such as cancer, heart disease, and opportunistic infections.”
The studies were supported by NIH grants DA025995, DA025201, DA05186, DA032776, and DA012756 (Clarke and colleagues), and DA024806 and DA032889 (Wachman and colleagues).
Sources
Clarke, T.-K., et al. Low frequency genetic variants in the μ-opioid receptor (OPRM1) affect risk for addiction to heroin and cocaine. Neuroscience Letters 542:71-75, 2013. Full text
Wachman, E.M.; Hayes, M.J.; Brown, M.S.; Paul, J.; Harvey-Wilkes, K.; Terrin, N.; Huggins, G.S.; Aranda, J.V.; Davis, J.M. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 309(17):1821-1827, 2013. Abstract
