The hypothesis that obesity and nicotine addiction have common genetic and biological roots is buttressed by a recent NIDA-supported study. Researchers showed that some gene variants that influence body mass index (BMI) also shape smoking behaviors.
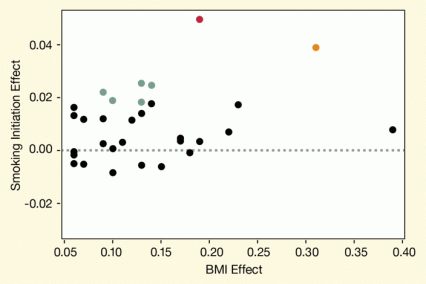 Figure. Several Gene Variants Affect Both BMI and Smoking Initiation Researchers found that SNPs that were associated with higher BMI in genome-wide association studies increased the likelihood that a person had initiated smoking. Dots representing SNPs are placed according to their effects on BMI and smoking initiation. The strengths of the associations with smoking initiation are indicated by color: red (p<0.0001), orange (p<0.001), green (p<0.05), and black (p>0.05).
Figure. Several Gene Variants Affect Both BMI and Smoking Initiation Researchers found that SNPs that were associated with higher BMI in genome-wide association studies increased the likelihood that a person had initiated smoking. Dots representing SNPs are placed according to their effects on BMI and smoking initiation. The strengths of the associations with smoking initiation are indicated by color: red (p<0.0001), orange (p<0.001), green (p<0.05), and black (p>0.05).
- Text description of Figure
-
The figure shows a scatter plot of the effects of single-nucleotide polymorphisms (SNPs) on body mass index (BMI) and initiation of smoking. The vertical (y)-axis represents the effect size of the SNPs on smoking initiation and the horizontal (x)-axis the effect of the SNPs on BMI. One SNP, indicated by a red dot, was most strongly associated with smoking initiation, indicated by the relatively largest effect size on this variable (>0.04) and by a very low p value of <0.0001, indicating a very high level of statistical significance; this SNP also had a moderately high effect on BMI (i.e., the effect size was approximately 0.2). Another SNP, indicated by an orange dot, had the second-highest effect (size of approximately 0.04) on smoking initiation, with a high level of statistical significance (p<0.001) and also with a relatively strong effect (>0.3) on BMI. Five SNPs, indicated as light green dots, had effect sizes on smoking that were moderately high (around 0.02) relative to those of the other SNPs, and these effects also reached statistical significance (p<0.05). They showed only relatively modest effects on BMI (>0.15). All other SNPs, indicated as black dots, had effect sizes of <0.02, which were not statistically significant (p>0.05) and had very variable effects on BMI, ranging in size from <0.1 to almost 0.4.
Dr. Thorgeir E. Thorgeirsson and colleagues at deCODE genetics/Amgen in Reykjavik, Iceland, examined associations between 32 BMI-influencing gene variants (called single nucleotide polymorphisms or SNPs, pronounced “snips”) and smoking (see Figure). Among 50,000 Icelanders who contributed DNA to the study, the SNPs, taken as a group, accounted for a small but significant fraction of individual differences in 2 smoking behaviors: initiation (whether someone had ever smoked) and the number of cigarettes smoked per day (CPD).
The researchers confirmed their results using DNA data from an additional 127,000 non-Icelandic individuals that were collected by 3 genomics consortia (ENGAGE, Tobacco and Genetics, and Oxford-GSK). They found that 11 of the 32 BMI-associated SNPs, considered individually, correlated with smoking at the p < 0.05 level of statistical significance. Of these, 9 were associated with smoking initiation, 2 with CPD, and 2 with both initiation and CPD.
Dr. Thorgeirsson and colleagues observed that most of the SNPs that associated with increased BMI also associated positively with smoking initiation or with higher CPD. These SNPs may influence both food intake and nicotine consumption.
Although the biological mechanisms that underlie those associations remain to be worked out, some of the SNPs are located within or near genes whose known biological roles provide potential clues. For example, considering the 2 SNPs most strongly associated with smoking:
- The top SNP for smoking initiation (rs10767664-A) occurs in the gene that codes for brain-derived neurotrophic factor (BDNF). BDNF participates in the biological processes underlying the initiation of new behaviors and habit formation. Hence, a SNP that alters BDNF function might influence the likelihood that someone will take up or persist in a behavior such as smoking.
- The top SNP for CPD (rs2867125-C) occurs close to the TMEM18 gene that codes for transmembrane protein 18. This protein may influence intake of both food and nicotine, as it is found throughout the brain, including in centers that affect appetite and reward.
Besides providing a glimpse at possible common origins of obesity and nicotine addiction, the study sheds light on a seeming paradox in the relationship between smoking and BMI: Smokers on average have a lower BMI than nonsmokers, but smokers who smoke more have a higher BMI than those who smoke less. Nicotine’s appetite-suppressing and metabolism-accelerating effects can account for the first observation. The study’s finding that some SNPs simultaneously promote increased BMI and nicotine consumption may partly explain the second.
This study was supported by NIH grants DA017932 and DA022522.
Source
Thorgeirsson, T.E.; Gudbjartsson, D.F.; Sulem, P. et al. A common biological basis of obesity and nicotine addiction. Translational Psychiatry 3: e308, 2013. Full text
