Varenicline (Chantix) helped outpatients with schizophrenia and bipolar disorder remain abstinent from smoking in an 18-month-long NIDA-supported clinical trial. The finding strengthens hope that pharmacotherapy can break nicotine’s especially tenacious hold on people with serious mental illness.
The need for effective anti-smoking treatments for people with severe mental illness is urgent and challenging. Severe psychiatric illness increases vulnerability to tobacco addiction and makes quitting harder. As a result, smoking prevalence among those with such illnesses has stayed roughly level despite evolving social attitudes and advances in treatment and prevention that have nearly halved smoking in the general population. The Substance Abuse and Mental Health Services Administration has estimated that 53 percent of the Nation’s adults with serious mental illness currently smoke, compared with 18 percent of adults in the overall population. Tobacco-related illnesses are the main reason why individuals with mental illnesses die an estimated 25 years sooner, on average, than those in the general population.
Abstinence Established and Sustained
Dr. A. Eden Evins at the Massachusetts General Hospital and Harvard Medical School in Boston and colleagues enrolled 203 smokers who were being treated for serious but stable mental illness into an open-label trial of varenicline and cognitive behavioral therapy (CBT). Eighty-seven (43 percent) had maintained continuous abstinence for 14 or more consecutive days at the end of the 12-week trial, and the researchers randomly allocated these participants to receive either long-term varenicline maintenance or placebo.
After 40 weeks of a maintenance treatment, 24 of 40 participants taking varenicline (60 percent) had not smoked for the past 7 days or longer, compared with 9 of 47 (19 percent) who received the placebo. Moreover, 18 (45 percent) of those treated with varenicline versus 7 (15 percent) of those given the placebo had sustained continuous abstinence throughout the 40 weeks (see Figure).
All of the study participants in the maintenance trial also received CBT. The behavioral therapy appeared to contribute to varenicline’s efficacy, which markedly declined when participants’ CBT schedule was reduced after week 12. From weeks 13 to 40 of the maintenance phase, with monthly rather than weekly or biweekly CBT, nearly one-third of those who had hitherto sustained continuous abstinence relapsed, even though they still took varenicline. In contrast, the researchers found no evidence that CBT yielded any positive effect when paired with placebo.
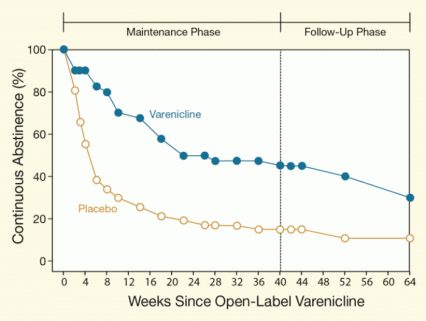 Figure. Varenicline Promotes Long-Term Smoking Abstinence Among People With Mental Illness Smokers who were abstinent at the end of a 12-week open-label trial of varenicline entered a 40-week maintenance trial. Those who received varenicline maintenance sustained higher rates of continuous abstinence than those who received a placebo. Both groups also received cognitive behavioral therapy (CBT). The varenicline advantage persisted through a 24-week follow-up phase after the medication and CBT were stopped.
Figure. Varenicline Promotes Long-Term Smoking Abstinence Among People With Mental Illness Smokers who were abstinent at the end of a 12-week open-label trial of varenicline entered a 40-week maintenance trial. Those who received varenicline maintenance sustained higher rates of continuous abstinence than those who received a placebo. Both groups also received cognitive behavioral therapy (CBT). The varenicline advantage persisted through a 24-week follow-up phase after the medication and CBT were stopped.
- Text description of Figure
-
The figure shows a line graph indicating continuous abstinence from smoking in study participants who had schizophrenia or bipolar disorder; the participants had quit smoking and received varenicline (blue line and symbols) or a placebo (beige line and symbols) during a maintenance phase. The vertical (y)-axis shows the proportion of abstinent study participants (in percent), and the horizontal (x)-axis shows the time (in weeks) since the participants had quit smoking. The study included 2 phases: One was the maintenance phase during which participants received varenicline or placebo (weeks 1 to 40) and the other a follow-up phase (weeks 41 to 64). The graph shows that although abstinence from smoking declined over time in both groups, among participants who received varenicline in the maintenance phase, more remained abstinent than in the placebo group. In the varenicline group, the proportion of abstinent individuals fell from 100 percent at the start of the maintenance phase to 45 percent at the end of the maintenance phase at week 40, while in the placebo group abstinence fell from 100 percent to 15 percent over the same time period. At the last follow-up, 64 weeks after all participants had abstained from smoking, 30 percent in the varenicline group were still abstinent versus 11 percent in the placebo group.
Pharmacological Function and Risk
Varenicline is a partial agonist at the same receptor to which nicotine binds, interacting with the receptor in a manner similar to, but more weakly than, nicotine. By occupying the receptor so that nicotine cannot access it, and by stimulating the receptor less, varenicline decreases nicotine reward and cravings.
A standard treatment course with varenicline consists of 12 weeks of the medication to establish abstinence, followed by an additional 12 weeks to secure long-term abstinence. Varenicline has been associated with adverse psychiatric effects in smokers without mental illness. However, although some patients in their trials received the medication for up to 52 weeks, Dr. Evins and colleagues did not observe any exacerbation of the participants’ established schizoid and bipolar symptoms. Nor did the medication appear to produce new psychiatric symptoms.
Nevertheless, the researchers caution that their study’s small size didn’t allow them to assess risks associated with longer-term varenicline use. They call for further studies with a larger number of participants over a longer abstinence period to determine the ideal length of treatment and to identify any risks associated with extended varenicline therapy.
This study was supported by NIH grant DA021245.
Source:
Evins, A.E.; Cather, C.; Pratt, S.A. et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: A randomized clinical trial. Journal of the American Medical Association 311(2):145-154, 2014. Full text
