A recent NIDA-funded clinical trial indicates that a significant portion of individuals who are addicted to opioid painkillers may initiate and maintain abstinence with a brief but intensive outpatient detoxification treatment followed by opioid antagonist therapy using naltrexone. Patients in the trial achieved higher abstinence rates than are typically obtained with detoxification regimens. The duration of a taper with buprenorphine/naloxone (Bp/Nx) was a determinative factor in patients’ success, with longer tapers yielding greater abstinence.
Dr. Stacey Sigmon and colleagues at the University of Vermont in Burlington enrolled 70 adults who were addicted to opioid painkillers into a double-blind, randomized clinical trial that involved a three-step detoxification process:
- Stabilization on a Bp/Nx (Suboxone) dosage that suppresses withdrawal symptoms, craving, and use of illicit opioid painkillers
- Gradual tapering of the Bp/Nx dose to zero over 1, 2, or 4 weeks
- Transition to the opioid antagonist naltrexone once a patient provides opioid-negative urine samples and reports no opioid use within the past 24 hours
The trial participants also received twice-weekly behavioral therapy using the evidence-based Community Reinforcement Approach and underwent thrice-weekly staff-observed urinalysis testing. Supplementary nonopioid medications were used as needed to treat breakthrough withdrawal symptoms.
The patients randomly assigned to the 4-week Bp/Nx taper provided the highest percentage of illicit opioid–free urine samples during the 12-week trial (see Figure). Of these 22 patients, 63 percent were abstinent at the 5-week mark, and 50 percent were still opioid-abstinent at the end of the 12-week trial. In contrast, 29 percent of each of the two groups receiving shorter tapers provided drug-free urine samples at 5 weeks, and 20 percent or less of each provided drug-free samples at 12 weeks. Similar findings were seen with adherence to naltrexone ingestion and treatment retention.
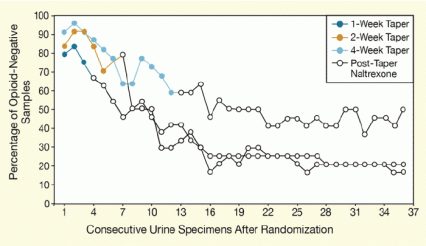 Figure. Longer Bp/Nx Taper Yields Greater Abstinence From Opioid Painkillers Patients randomly assigned to taper off Bp/Nx over 4 weeks provided more opioid-free urine samples than patients assigned to 1- or 2-week tapers. All patients received naltrexone maintenance treatment after the end of their taper and twice-weekly behavioral counseling throughout the study.
Figure. Longer Bp/Nx Taper Yields Greater Abstinence From Opioid Painkillers Patients randomly assigned to taper off Bp/Nx over 4 weeks provided more opioid-free urine samples than patients assigned to 1- or 2-week tapers. All patients received naltrexone maintenance treatment after the end of their taper and twice-weekly behavioral counseling throughout the study.
- Text Description of Graphic
-
The figure shows a line graph indicating the percentages of opioid-negative urine samples under three different regimens of tapering users of opioid painkillers with buprenorphine/naloxone (Bp/Nx) followed by maintenance treatment on naltrexone. The vertical (y)-axis shows the percentage of opioid-negative samples the patients submitted and the horizontal (x)-axis the number of consecutive urine specimens after the patients had been randomized to one of the three tapers. One group of patients, represented by dark blue lines and symbols for the length of the taper and by a black line thereafter, were randomized to a Bp/Nx taper for 1 week; another group, represented by orange lines and symbols for the length of the taper and by a black line thereafter, were randomized to a Bp/Nx taper for 2 weeks; and a third group, represented by light blue lines and symbols for the length of the taper and by a black line thereafter, were randomized to a Bp/Nx taper for 4 weeks. Patients randomized to Bp/Nx tapering for 4 weeks provided the highest percentage of opioid-negative urine samples—about 60 percent at the end of the taper and about 50 percent at the end of the 12-week trial. Patients in the 1- and 2-week taper regimens provided similar percentages of opioid-free urine samples, and after the tapers ended, these percentages were almost always lower than from those who had undergone the 4-week taper. And by the end of the trial, the percentages of opioid-free urine samples from the patients in the 1- and 2-week tapers had dropped down to 20 percent or less.
Working Parts
Although many patients who are dependent on opioids will benefit from long-term maintenance therapy with methadone or buprenorphine, the Vermont study suggests that there may be a meaningful subset of individuals who can obtain good outcomes with more time-limited approaches, Dr. Sigmon says.
“Detoxification is typically associated with high relapse rates and return to opioid abuse,” Dr. Sigmon says. “However, some data also suggest that if you do it right, outpatient detoxification can be effective. The inclusion of naltrexone therapy is likely extremely important to help prevent resumption of illicit opioid use following detoxification.”
Dr. Sigmon notes that outpatient detoxification may be particularly appropriate for patients who present for treatment with less severe opioid dependence. In this trial, consistent with previous research, stabilization on a lower dose of buprenorphine—an indicator of less severe dependence—was associated with a favorable treatment response. “This finding may hold particular relevance for prescription opioid abusers, many of whom are younger and have briefer histories of opioid dependence, less severe other drug use, less IV use, and greater psychosocial stability than past generations of primary heroin abusers,” says Dr. Sigmon.
Although their study design did not permit them to measure the impacts of the treatment regimen’s intensive behavioral therapy and naltrexone maintenance, the researchers believe that both were instrumental to their patients’ positive outcomes. The behavioral therapy, delivered by master’s-level therapists, included counseling on how to handle withdrawal and avoid relapse, strengthen social networks, and find healthy recreational activities. The patients were also offered individually tailored sessions focused on their particular needs, from employment to managing depression.
Naltrexone is a nonopioid medication that blocks the receptors where opioids bind and exert their effects. A patient undergoing treatment for opioid addiction who slips and takes an opioid drug doesn’t get the expected high or euphoria. “I suggest that patients view naltrexone as an insurance policy,” Dr. Sigmon says. “It can prevent a single lapse from turning into a full-blown relapse. The idea is, if you don’t have any drug effect, why spend $100 for 100 milligrams of OxyContin?” Naltrexone can be taken long term to prevent resumption of opioid use after detoxification.
The Vermont team’s finding that longer Bp/Nx tapers enhance patients’ outcomes is consistent with some, although not all, previous studies on detoxification. Dr. Sigmon says that more gradual Bp/Nx tapering may more completely suppress opioid withdrawal symptoms, thereby reducing patient discomfort and risk for relapse to opioids.
Dr. Will Aklin, acting chief in NIDA’s Behavioral and Integrative Treatment Branch, says that the current rates of opioid painkiller misuse and dependence have strained treatment resources and challenged researchers and clinicians to fashion effective therapies for a population that is at significant risk for overdose and death. The Vermont team’s findings represent a meaningful advance toward meeting that challenge, Dr. Aklin says.
He adds that future studies might investigate how innovative approaches for delivering medications and new technology to remotely support and monitor abstinence could complement the intensive detoxification regimen developed by Dr. Sigmon’s team. “This is especially important in rural or other resource-constrained settings, where community-based treatment centers can benefit from such complementary programs,” says Dr. Aklin.
This study was supported by NIH grants DA019989 and DA007242.
Source:
Sigmon, S.C.; Dunn, K.E.; Saulsgiver, K. et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 70(12):1347-1354, 2013. Full text
See Also:
Ruetsch, C. Treating prescription opioid dependence. JAMA, 312(11):1145-1146, 2014. Abstract
