 Dr. Marco Diana
Dr. Marco Diana Dr. Diana Martinez
Dr. Diana MartinezA few years ago, Dr. Marco Diana and Dr. Diana Martinez each independently decided to investigate whether applying powerful electromagnetic fields to patients’ brains can alleviate cocaine addiction. The technology they would use—transcranial magnetic stimulation (TMS)—had already produced promising results in other mental health disorders, including depression, nicotine addiction, and schizophrenia. However, much remains unknown about its effects and capabilities.
Dr. Diana, of the University of Sassari, Italy, began a pilot clinical trial of TMS. At the time, he hoped that by now he would have shown that TMS is worthy of a full-scale trial that might establish it as a solidly research-proven therapy. Instead, his pilot trial is only half finished. The reason for the long delay could be a case study in how stigmatized people may become suspicious even of those who want to help them.
Dr. Martinez, of Columbia University Health Center, in New York City, started later than Dr. Diana. Her initial goal was to rapidly identify which, among hundreds of possible ways to deploy the TMS coil (delivery device; see Figure 1), has potential to be most effective. Today, she feels she is closing in on an answer.
In this article, Dr. Diana and Dr. Martinez share their motives and reasons for researching TMS and relate their efforts so far. Future articles will follow the researchers as they work through the challenges of adapting a new technology to address the urgent and stubborn problem of cocaine addiction.
NIDA Notes’ Narratives of Discovery series present drug abuse research as it actually happens. Readers accompany researchers as they conceptualize problems and apply the tools and methods of science to solve them, bringing to bear their research experience, savvy, and intuitions. We hope to convey the idealism, determination, and excitement with which scientists strive to reduce the human toll of drug use and addiction.
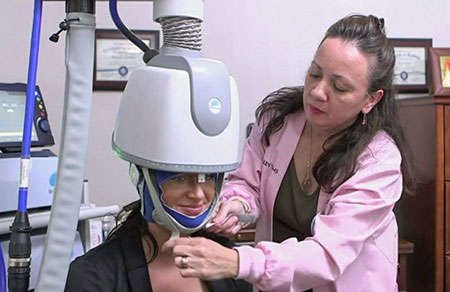 This photo is courtesy of Brainsway Figure 1. The TMS Coil Projects an Electromagnetic Field. This causes electrical current to flow through a targeted brain region or circuit. Depending on the coil settings, the field will either increase the firing rate of neurons in the targeted region (i.e., stimulate or activate the region) or decrease the firing rate (i.e., inhibit or deactivate the region).
This photo is courtesy of Brainsway Figure 1. The TMS Coil Projects an Electromagnetic Field. This causes electrical current to flow through a targeted brain region or circuit. Depending on the coil settings, the field will either increase the firing rate of neurons in the targeted region (i.e., stimulate or activate the region) or decrease the firing rate (i.e., inhibit or deactivate the region).Magnetic Attraction
TMS produces therapeutic effects by stimulating underperforming brain areas to higher levels of metabolic activity. The hypothesis that TMS can alleviate addiction rests on observations that in people who are addicted, the prefrontal cortex (PFC) and regions that use the neurotransmitter dopamine are just such underperforming areas. According to theory, these weaknesses contribute to the poor decision-making and compulsive focusing on drugs that are hallmarks of addiction.
Dr. Diana and Dr. Martinez turned their attention to TMS after having each spent years seeking to develop a medication for cocaine addiction. In deciding to change course, they were influenced by two potential advantages of the new technology.
First, TMS may avoid the risk for side effects that is associated with most medications. Dr. Diana, a research pharmacologist, explains, “Medications circulate throughout the entire brain and body. In many instances, the pharmacological activity that confers a therapeutic effect in one brain area or body part causes undesirable effects elsewhere. With TMS, in contrast, the clinician can direct an electromagnetic field precisely to the brain area he wants to stimulate.”
Second, both researchers had become impatient with the slow pace of medications development. As TMS has already been proven safe for people, it may offer a short cut to the goal of a proven, clinic-ready medication for cocaine addiction.
Dr. Martinez felt this second advantage acutely. An expert in brain imaging, she contributed to the research linking addictive drugs to reduced PFC and dopamine activity. At the time she performed those studies, she intended to parlay her findings into the development of medications that could re-energize the brain areas that are critically compromised in addiction. Instead, she found herself stalemated. She identified several compounds that she still feels might be effective, but in each case, she was unable to obtain permission to test the compound from the pharmaceutical company that owned the patent on it.
“I’ve spent a lot of time imaging people’s brains, and these data have led to good ideas for medications,” she says. “But we can’t get our hands on them. You’re just beating your head against the wall when it comes to pharmaceutical companies and addiction.”
The two researchers are evaluating different approaches to TMS. Dr. Diana is focusing the TMS field on patients’ dorsolateral PFC to try to reinvigorate the dopamine system. Dr. Martinez is directing the field more deeply and less specifically, with the aim of broadly strengthening neuronal signaling in the PFC. By attacking the problem from different angles, the researchers increase their chances not only of discovering an effective TMS protocol for addiction, but of finding an optimal one.
The Case of the Suspicious Patients
In Dr. Diana’s trial, patients check into an outpatient clinic at SerT hospital in Marsciano, Italy, for a month. Every other day they go into a treatment room, where two-thirds receive TMS and the remainder sham TMS. Neither the researchers nor the patients know who is getting which treatment. All patients also receive supportive counseling.
The test for TMS will be whether the patients who receive it reduce their cocaine use more than those who get the sham. Dr. Diana and colleagues Dr. Mariano Pedetti and Dr. Corinna Bolloni interview each patient and analyze his or her hair for cocaine metabolites upon hospital entry, at the end of treatment, and every 3 months for a year.
The TMS treatments go remarkably smoothly, Dr. Diana reports. “Patients sit in a chair. They are fully conscious, completely normal. We position the coil over both dorsolateral prefrontal cortices (see Figure 2A) and put earplugs in their ears. They take the treatment for 15 minutes. They stand up and leave. A few have reported mild dizziness, problems in the ears, but we haven’t seen any painful or serious side effects.”
The follow-up assessments made to date give grounds for optimism: Most patients have submitted cocaine-negative hair samples after the treatment.
Encouraged by these observations, Dr. Diana is eager to reach the next milepost in his study. When he has treated 30 patients, he will break the treatment code and learn which ones have received TMS and which sham. Although he calculates that he needs to treat 60 patients to provide statistically convincing support for advancing TMS to the next testing stage, this midpoint analysis will give him an interim read on how the patients are faring.
To Dr. Diana’s surprise and chagrin, however, getting to 30 patients is taking a long, long time. In the 3 years since his team began advertising for trial participants—in newspapers, on the internet, and elsewhere—only 20 individuals have signed on and undergone the treatment. Many more have declined.
The reason, Dr. Diana says, is mistrust. “People are skeptical. When I describe the treatment to them, they say, ‘I know, you want to give me electroshock.’ The source of their fear is probably popular beliefs or legends that were inspired by the psychiatric use of electroshock in the early 1900s.”
That people who are desperate for help forgo TMS because of misplaced suspicion is especially poignant, Dr. Diana says, in light of how easily people who agree to the treatment have been tolerating it. He points to his own experience of donning the TMS coil. “I tried it because I was curious,” he reports. “I felt a strange sensation in my ears. It was like a fly or a bee buzzing in my ear canal for 15 or 20 minutes. There was no pain, nothing annoying.”
Could Dr. Diana speed up recruitment by modifying his outreach materials to address, and hopefully allay, potential participants’ fears? That would break a cardinal rule of clinical trials, Dr. Diana says, which is that all participants must be treated exactly the same. The patients who would respond to a changed recruitment interview might differ from those who already underwent TMS in ways that would affect their responses to the intervention. If that happened, it would confound the researchers’ ability to interpret their results.
Hopeful and determined, Dr. Diana has settled in for the long haul. He estimates that he will be ready to break the code in the spring of 2016.
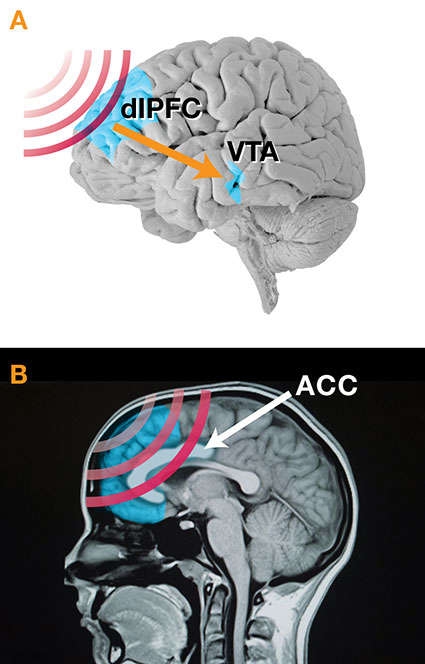 Figure 2A. To restore lost activity in the dopamine system, Dr. Diana is focusing TMS on patients' dorsolateral prefrontal cortex (dlPFC). TMS-stimulated neurons in the dlPFC, in turn, stimulate the ventral tegmental area (VTA), increasing the firing rate of specialized neurons that produce the neurotransmitter. Direct targeting of the VTA with TMS is not feasible because current coils cannot focus sharply that deeply into the brain.
Figure 2A. To restore lost activity in the dopamine system, Dr. Diana is focusing TMS on patients' dorsolateral prefrontal cortex (dlPFC). TMS-stimulated neurons in the dlPFC, in turn, stimulate the ventral tegmental area (VTA), increasing the firing rate of specialized neurons that produce the neurotransmitter. Direct targeting of the VTA with TMS is not feasible because current coils cannot focus sharply that deeply into the brain.Figure 2B. To restore lost vigor throughout the PFC, Dr. Martinez is using the TMS coil to cast a relatively softly focused electromagnetic field deeply into that brain area. The field centers on the anterior cingulate cortex (ACC), but also stimulates the medial PFC, parts of the dlPFC, and perhaps other subregions.
- Text Description of Graphic
-
The upper panel (A) shows a parasagittal view of a brain with its frontal part oriented to the left. The dorsolateral prefrontal cortex, an area about one-tenth of the total area in this graphic, is located at approximately 10 o’clock and near the top of the first quadrant and is shown in light blue. Red wave forms indicating TMS are directed at the dlPFC, and its direct stimulation results in indirect stimulation, which is indicated by an orange arrow, of the ventral tegmental area, a small region also highlighted in light blue and located approximately in the lower one-third of the border between the third and fourth quadrants of the brain.
The lower panel (B) shows an MRI image of a skull including the brain in the sagittal plane. Red-wave forms, indicating TMS, are passing through the frontal part of the brain (on the left), which contains the prefrontal cortex (colored in light blue), and are most intensely focused on the anterior cingulate cortex (ACC), a banana-shaped structure in the central anterior region of the brain.
A Mixed Result
Having come to TMS later than Dr. Diana, Dr. Martinez is at an earlier stage in her investigations. Because TMS is so new, researchers must learn how to deploy their coils through trial and error. “With TMS there are countless coil settings that you can use,” Dr. Martinez explains. “Our goal right now is to fast-screen different settings to identify which combinations might work in the clinic. Then we’ll take what we learn and use it in a clinical trial.”
Dr. Martinez recruits cocaine users who don’t wish to quit but agree to participate in her study. They check into the hospital and undergo 3 weeks of daily TMS. Before and after the TMS course, they are given a choice between money and self-administering cocaine in a controlled laboratory setting. If they opt for less cocaine after the TMS course than they did before, then the settings used may be worth testing in a clinical trial. Otherwise, Dr. Martinez scratches off those settings and tries others.
For her initial screens, Dr. Martinez selected a frequency setting of 10 Hz. Frequency is a key TMS setting. Depending on the frequency used, TMS magnetic fields can either stimulate or inhibit neural activity—mildly, moderately, or intensely. Success in any treatment application depends on finding a frequency that compensates for the hyper- or hypo-activity that’s causing the patient’s problem.
The initial screens generated a conundrum. Dr. Martinez recounts, “A few participants completed the TMS course. These people did well, using less cocaine after the treatment than they had before. However, most participants dropped out after the first treatment.
“The dropout rate was especially striking because these people were highly motivated. They had participated in many studies. They usually would try to stay in the hospital for an extra day at the end of a study, to collect another day’s compensation. But in this case, after the very first TMS session, they literally were saying, ‘I’m leaving. I’m leaving the hospital now.’
“It’s remarkable to have an effect like that. People came in to the study counting up how much money I was going to owe them for their participation. They were sure this was going to be a breeze. And the next thing we knew, they were leaving. And they were adamant that nothing was going to change that decision.”
After 7 of 11 participants pulled out, Dr. Martinez called a halt. “Obviously,” she says, “a treatment that 60 to 70 percent of patients can’t tolerate is going to be problematic.”
Adjust and Readjust
Dr. Martinez and her team pondered what to do next. The participants’ reports of why they rejected TMS provided scant clues. “Their reasons weren’t clear,” Dr. Martinez says. “People said the procedure was sort of painful, but something else seemed to be going on as well. The treatment seemed to provoke this incredible anxiety.”
One possible adjustment was to change the frequency of TMS. In choosing 10 Hz, Dr. Martinez had hypothesized that high-frequency stimulation would be needed to reverse cocaine’s blunting of activity in the PFC. Other researchers, including Dr. Diana, had applied this frequency to patients without problems. However, cocaine-addicted patients in one study had complained of pain when exposed to TMS at 10 Hz.
Dr. Martinez decided to try a lower frequency. She recruited a new cohort of cocaine users and screened them, using all the same procedures, at 1 Hz.
“We were feeling our way forward at that point,” says Dr. Martinez. “There was no real scientific reason to think that TMS at 1 Hz should be effective. It was only that 10 Hz seemed to have been too intense, and 1 Hz was less than 10 Hz.”
As she suspected might happen, the screen at 1 Hz produced results opposite to those of the initial screen. The participants tolerated TMS well, but did not benefit. Although many reported feeling drowsy, none exhibited distress, and they did not reduce their cocaine use.
Dr. Martinez considered what to do next. One option was to try a TMS frequency intermediate between 1 Hz and 10 Hz. Perhaps she could find a sweet spot that would as effective as 10 Hz and as tolerable as 1 Hz. A colleague referred her to a study that suggested that the 4-8 Hz range might be ideal. The researchers who conducted the study had concluded that the anterior cingulate cortex (see Figure 2B) “works best” at those frequencies.
Instead, Dr. Martinez elected to screen again with the 10 Hz frequency. This time, however, she would change other aspects of her TMS protocol to try to make the treatment comfortable for patients.
Stay Tuned
The next article in this Narrative of Discovery series will relate how Dr. Martinez adjusted her protocol and her rationale, and how patients responded. Future articles will check in with Dr. Diana as he works toward and beyond breaking the code on his pilot clinical trial.
Both researchers feel they are bringing closer a day when TMS finds a place among proven effective therapies for addiction. Dr. Diana says, “Thanks especially to work by Dr. Nora Volkow, Dr. Martinez, and our own basic science work, we now know a great deal about the neurobiology of cocaine addiction. However, we still have virtually no therapies. The promise of TMS is great. And, it will keep increasing as coil technology progresses, making it possible to focus and calibrate the amount of stimulation more and more precisely.”
