In the first long-term follow-up of patients treated with buprenorphine/naloxone (Bp/Nx) for addiction to opioid pain relievers, half reported that they were abstinent from the drugs 18 months after starting the therapy. After 3.5 years, the portion who reported being abstinent had risen further, to 61 percent, and fewer than 10 percent met diagnostic criteria for dependence on the drugs.
“Our findings are cause for optimism for these specific patients,” says Dr. Roger Weiss, of McLean Hospital and Harvard Medical School, who co-led the study. The findings indicate that two factors strongly influence patients’ chances for recovery from addiction to pain relievers:
- At each follow-up, patients who told interviewers that they were currently engaged in opioid agonist therapy were much more likely to report that they were abstinent than those who were not using the medications.
- Patients who had ever used heroin had poorer odds of overcoming their pain reliever addiction than those who had never done so.
Treatment and Recovery
Dr. Weiss and Dr. Jennifer Sharpe Potter, associate professor of psychiatry and assistant dean for research and student programs in the School of Medicine at the University of Texas Health Science Center at San Antonio, and colleagues affiliated with the NIDA Clinical Trials Network, followed the progress of patients who had participated in the Prescription Opioid Addiction Treatment Study (POATS). POATS comprised two phases. In the first, 653 patients received Bp/Nx for 2 weeks to stabilize their opioid exposure and suppress craving and withdrawal symptoms, followed by a 2-week taper off the medication and a 2-month follow-up. In the second phase, patients who had reverted to use of opioid pain relievers during the first phase were offered a longer course of Bp/Nx, consisting of 12 weeks of stabilization followed by a 4-week taper and 2-month follow-up. The POATS researchers reported that:
- A small minority (6.6 percent) of patients successfully maintained abstinence from opioid pain relievers throughout the phase-one brief stabilization, taper, and follow-up
- Nearly half of the 360 patients who entered phase two achieved abstinence or near-abstinence from opioids during their last 4 weeks of Bp/Nx stabilization
- However, fewer than 10 percent of the patients in the second phase were still doing well at the end of the 2-month follow-up.
To assess long-term outcomes after Bp/NX stabilization and taper, the researchers interviewed 252 of the 653 original POATS participants by telephone 18 months after the start of the trial, and more than 300 participants after 30 and 42 months. The portions of participants who reported that they had been abstinent for 30 days, and whose responses indicated that they were not currently dependent on pain relievers, respectively, were (see Figure):
- At 18 months, 51.2 percent and 83.7 percent
- At 30 months, 63.5 percent and 88.5 percent
- At 42 months, 61.4 percent and 92.2 percent.
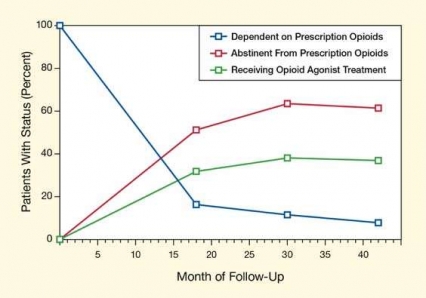 Figure. Abstinence Rate Exceeds 60 Percent in Long-Term Follow-Up of Medication-Assisted Therapy for Dependence on Opioid Pain Relievers Dependence on pain relievers dropped below 20 percent at 18 months, and below 10 percent at 42 months, after patients were stabilized on, and then tapered off, Bp/Nx. At all three follow-up points, patients who were currently engaged in opioid agonist therapy had markedly higher odds of positive outcomes.
Figure. Abstinence Rate Exceeds 60 Percent in Long-Term Follow-Up of Medication-Assisted Therapy for Dependence on Opioid Pain Relievers Dependence on pain relievers dropped below 20 percent at 18 months, and below 10 percent at 42 months, after patients were stabilized on, and then tapered off, Bp/Nx. At all three follow-up points, patients who were currently engaged in opioid agonist therapy had markedly higher odds of positive outcomes.
- Text Description of Graphic
-
The figure shows a line graph indicating dependence on or abstinence from prescription opioids and involvement in opioid agonist treatment among the patients in the study cohort receiving Bp/Nx treatment. The vertical (y)-axis shows the proportion of patients meeting the criteria for the three specified conditions in percent, and the horizontal (x)-axis shows the month of follow-up. At month 0, all (100 percent) patients in the study were dependent on prescription opioids; this percentage dropped steeply to less than 20 percent at follow-up month 18 and then gradually declined to less than 10 percent at month 42. Abstinence from prescription opioids among the patients rose from 0 percent of patients at month 0 to about 50 percent at follow-up month 18, peaked to more than 60 percent at month 30 and remained at about 60 percent at month 42. The proportion of patients receiving opioid agonist treatment rose from 0 percent at month 0 to about 30 percent at follow-up month 18, peaked at just below 40 percent at month 30, and remained roughly the same at month 42.
An Efficacious Medication
Bp/Nx, an opioid partial agonist–antagonist, prevents cravings, mitigates withdrawal symptoms, and blocks the effects of other opioid drugs. The new study affirms others that have found that ongoing use of opioid agonist medications enhances many patients’ chances of recovering from opioid addiction.
Thus, whereas 49.2 percent of POATS participants who received 12 weeks of Bp/Nx were abstinent at the end of this treatment period, the abstinence rate rapidly collapsed, to 8.6 percent, within 2 months after patients were tapered off the medication. The 18-month follow-up found many patients currently or recently re-engaged in opioid agonist therapy, and the abstinence rate rebounded to 51.2 percent. Patients who were using opioid agonist medications at the 18-month interview were more than twice as likely to report abstinence as those who were not (80.0 percent versus 36.6 percent). At the 42-month interview, the advantage associated with current opioid agonist treatment had narrowed, but was still large (79.6 percent versus 50.8 percent).
The researchers noted that the patients who succeeded with the initial short 4-week Bp/Nx stabilization and taper in the POATS trial were an exception to the general observation that current opioid agonist therapy is an important asset for maintaining abstinence. Almost all of them stayed abstinent throughout the 18-month follow-up without any further opioid agonist therapy.
Bp/Nx appeared to be especially beneficial for patients with depression. “Patients with a lifetime history of major depressive disorder were nearly twice as likely as patients without such a history to have a good outcome during the 12-week Bp/Nx treatment,” Dr. Weiss reports. Moreover, patients who had more severe depressive symptoms at the start of the study reported more participation in opioid agonist therapy during the follow-up, potentially enhancing their chances for success. These findings accord with other researchers’ suggestions that Bp/Nx may have antidepressant effects. Dr. Weiss also notes that patients with depression may be more motivated to make life changes to alleviate psychological distress.
Predictors of Poor Outcomes
A history of heroin use was associated with poor outcomes throughout the POATS trial and follow-up, in line with earlier findings by Dr. Potter’s team (see Study Points to Individualized Therapy for Opioid Addiction). Consistent with other reports indicating that some users of prescription opioids switch to heroin, 1 in 10 users who had not used heroin before the start of the study reported injecting heroin by month 30.
Greater pain severity at the start of the POATS trial predicted prescription opioid dependence at month 18, a finding that diverges from earlier observations that pain severity is unconnected with outcome in addiction treatment. However, at month 42, many patients reported less pain and also better health, outcomes the researchers say may have been due to improvements accompanying reductions in substance use.
“Our results represent an important first step toward understanding the course of dependence on opioid pain relievers, and for identifying factors associated with longer-term recovery,” Dr. Potter says. She and her colleagues say that their findings constitute strong evidence that treatment with opioid agonist medications, such as Bp/Nx, can be effective tools for combating today’s epidemic of pain reliever misuse and dependence.
This study was supported by NIH grants DA020024, DA02297, DA15831, and DA022288.
Sources:
Potter, J.S.; Dreifuss, J.A.; Marino E.N. et al. The multisite prescription opioid addiction treatment study: 18-month outcomes. Journal of Substance Abuse Treatment (48)1:62-69, 2015. Abstract
Weiss, R.D.; Potter, J.S.; Griffin, M.L. et al. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug and Alcohol Dependence 150:112-119, 2015. Abstract
