A protein that is naturally present in the brain reduces laboratory animals' attraction to environments in which they have experienced cocaine's effects. The recent finding could point the way to new treatments to help people overcome addiction to cocaine and perhaps to other drugs.
Dr. Collin Kreple, Dr. John Wemmie, and colleagues at the University of Iowa and U.S. Department of Veterans Affairs Medical Center, Iowa City, previously showed that the protein, called acid-sensing ion channel 1A (ASIC1A), supports some types of learning and memory. In a new NIDA-supported study, they found, to their surprise, that ASIC1A exerts an opposite, inhibiting effect on cocaine-related learning and memory. These contrasting effects suggest that medications that increase ASIC1A might block the formation of powerful drug-context associations that promote cocaine use and relapse, while leaving intact—or even improving—other kinds of memory.
Reinforcement and Learning
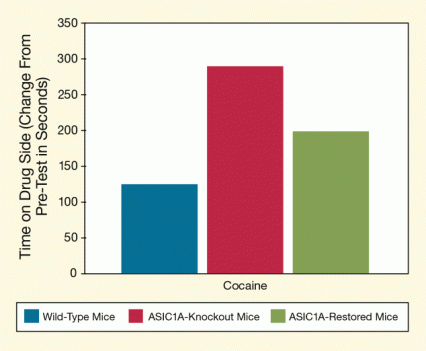 Figure 1. ASIC1A Dampens Animals’ Preference for Cocaine-Related Environments After receiving cocaine in a distinctively marked chamber, mice spent more time in that chamber than in another where they had not received the drug. This preference was less strongly marked in mice with normal ASIC1A (blue bar) than in mice that were genetically modified to lack ASIC1A (ASIC-knockouts, red bar). When researchers restored ASIC1A to near normal levels in the nucleus accumbens (green bar), the animals exhibited place preference similar to that shown by normal mice, and less than that shown by unrestored knockouts. The results suggest that ASIC1A may limit vulnerability to cocaine relapse by making the brain less sensitive to drug-related learning and rewards.
Figure 1. ASIC1A Dampens Animals’ Preference for Cocaine-Related Environments After receiving cocaine in a distinctively marked chamber, mice spent more time in that chamber than in another where they had not received the drug. This preference was less strongly marked in mice with normal ASIC1A (blue bar) than in mice that were genetically modified to lack ASIC1A (ASIC-knockouts, red bar). When researchers restored ASIC1A to near normal levels in the nucleus accumbens (green bar), the animals exhibited place preference similar to that shown by normal mice, and less than that shown by unrestored knockouts. The results suggest that ASIC1A may limit vulnerability to cocaine relapse by making the brain less sensitive to drug-related learning and rewards.
- Text Description of Figure 1
-
The figure shows a bar graph indicating the increase in the length of time mice spent in the chamber of a cage where they had received cocaine for three groups of animals: wild-type, ASIC1A-knockout, and ASIC1A-restored mice. The horizontal (x) axis shows the groups of mice, and the vertical (y) axis shows how much longer the animals spent in the drug chamber after cocaine exposure (as the change in time before and after the drug exposure in seconds). As indicated by blue bars, the wild-type mice spent about 100 seconds longer in the drug chamber, whereas the ASIC1A-knockout mice (indicated by red bars) spent almost 300 seconds longer in that chamber. As shown by a green bar, restoring the ASIC1A channel in ASIC1A-knockout mice reduced the differential in the time spent in the drug chamber to under 200 seconds.
The researchers repeatedly exposed mice to cocaine in a distinctively marked test chamber, and then gave the animals a choice: They could hang out either in that chamber or in another where they had not received cocaine. Normal (“wild-type”) mice and mice that were genetically altered to lack ASIC1A both favored the chamber where they had received cocaine, and spent the majority of their time there. However, the normal animals exhibited this preference less markedly (see Figure 1).
The finding suggested that ASIC1A inhibits the pleasure obtained from taking cocaine or the learning processes required to associate that pleasure with the environments in which it is experienced. To further establish the link between ASIC1A and drug-related behavior, the researchers injected the protein into the nucleus accumbens (NAc) of the genetically altered mice and repeated the chamber-preference test. With their ASIC1A levels thus raised to near those of wild-type mice, the genetically altered animals now spent less time in the cocaine-associated chamber than did a control group of still-ASIC1A-deficient mice. This observation confirmed that that protein exerts a dampening effect on cocaine-seeking behavior.
The researchers then turned to another animal, the rat, to assess ASIC1A’s effect on cocaine reinforcement. In these experiments, rats self-administered 30-milligram doses of cocaine in test sessions that took place before and after the researchers increased ASIC1A in their NAc. The animals self-administered less cocaine in the second session, after they received the ASIC1A supplement, indicating that the protein reduces the drug’s reinforcing effect.
The researchers concluded that, in animals, ASIC1A suppresses two important drivers of cocaine addiction and relapse: cocaine reinforcement and drug-association learning. Moreover, raising ASIC1A levels in normal animals can strengthen these effects.
Location, Location
Drs. Kreple and Wemmie were surprised by their results. They had expected to find that ASIC1A strengthens, rather than weakens, cocaine reinforcement and cocaine-related associations. Such findings would have paralleled previous observations by the two researchers and others that ASIC1A promotes some types of learning. For example, ASIC1A promotes associations between fearful experiences and the places where they were experienced. Similarly, normal animals solve the Morris water maze, a test that requires them to remember spatial relationships, more readily than ASIC1A-deficient animals.
The discrepant findings suggest that ASIC1A exerts opposite effects in different brain regions—that it promotes memory and learning in the amygdala and hippocampus (sites for fear-based associations and spatial memory) and inhibits them in the NAc (the main site for learning related to rewarding experiences, including those resulting from drug exposure). Consistent with this hypothesis, a comparison of NAc neurons in normal and in ASIC1A-deficient mice found structural differences that were, indeed, the reverse of differences that occur between hippocampal neurons of normal and ASIC1A-deficient mice. Dr. Wemmie and colleagues observed that NAc neurons in normal mice featured:
- Fewer dendritic spines
- Lower availability of one subtype of receptor for the neurotransmitter glutamate (i.e., α-amino-3-hydroxy-5-methyl-4-isoxazole propionate [AMPA] receptors that lack the unit GluA2)
- A lower ratio of available AMPA receptors to another type of glutamate receptor (N-methyl-d-aspartate [NMDA])
These differences were all consistent with ASIC1A in the NAc limiting cocaine reward and inhibiting cocaine-related learning and memory. An increase in dendrites, as seen in the ASIC1A-deficient mice, has been linked to animals’ increased preference for environments that they associate with cocaine. Alterations in glutamate receptor quantity and composition such as those seen in the ASIC1A-lacking mice have been associated with enhanced responsiveness to the drugs’ reinforcing effects. These receptor changes also have been shown to underlie memory associations linking rewarding experiences to the places in which they occur.
Activation by Protons
In further experiments, Dr. Wemmie and colleagues added detail to our understanding of ASIC1A’s activity and effects on neurons in the NAc. These investigations identified a likely starting point and mechanisms for ASIC1A’s effects on NAc neuron structure and responses to cocaine.
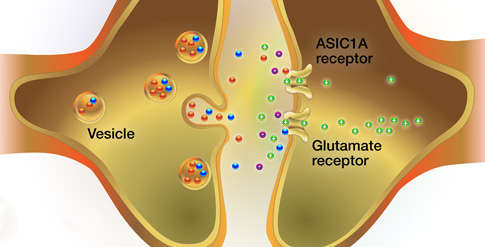
Previous research had shown that ASIC1A is present on neurons throughout the brain. The protein resides on the neuron postsynaptic membrane, which is the part of the neuron surface that receives excitatory and inhibitory signals from neighboring neurons. The ASIC1A lumen opens and allows calcium ions to pass from the neuron exterior to the neuron interior when the pH of the surrounding environment is low (i.e., acidic). However, the exact circumstances that trigger ACIS1A activation, and how the resulting changes in ion flow affect the neuron, remained unknown.
Dr. Wemmie and colleagues hypothesized that free protons activate ASIC1A, based on two premises. First, other researchers had reported that when a presynaptic “sending” neuron releases neurotransmitter, it also releases free protons into the synaptic space between itself and the postsynaptic “receiving” neuron. Second, free protons lower pH in tissues.
The researchers confirmed their hypothesis by using tissue slices from the core region of the NAc of normal mice. In these experiments, they monitored electric current in postsynaptic neurons while stimulating release of neurotransmitters from presynaptic neurons. By bathing the tissue slices in compounds that block postsynaptic currents arising from other sources, the researchers isolated a small current that was attributable, by process of elimination, to activated ASIC1A. This current occurred concurrently with the release of the neurotransmitter glutamate from the presynaptic neuron. It was absent in NAc core tissue from ASIC1A-deficient mice, but appeared when the researchers chemically added ASIC1A to the tissue.
Pathway to Protection
Taken together, the Iowa researchers’ findings outline a pathway linking ASIC1A to reduced cocaine-related learning and behavior:
- Presynaptic neurons in the NAc core release glutamate and, simultaneously, release protons into the synapse.
- The glutamate and protons activate glutamate receptors and ASIC1A, respectively, on postsynaptic medium spiny neurons.
- Positive ions flow through the activated glutamate receptors and ASIC1A.
- The positive ions induce electric current in the postsynaptic neurons, with about 95 percent of the current contributed by the glutamate receptors and about 5 percent contributed by ASIC1A.
- The current induces mechanisms inside the neurons that, ultimately, result in structural differences that inhibit behavioral responses to cocaine (see Figure 2).
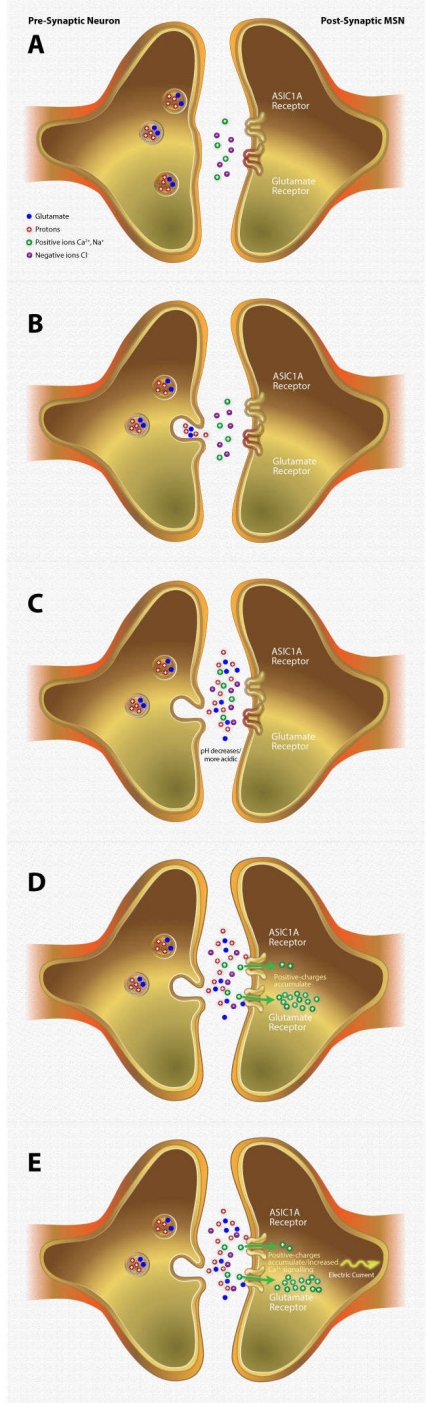
Figure 2. How ASIC1A Diminishes Responses to Cocaine
Panel A: Vesicles in presynaptic neurons contain the neurotransmitter glutamate and protons. The synapse between the presynaptic neuron and postsynaptic medium spiny neuron (MSN) is normally at a slightly alkaline pH.
Panel B: When the presynaptic neuron is stimulated, the vesicles release their contents into the synapse.
Panel C: The released protons lower the synaptic pH.
Panel D: Glutamate binds to and activates glutamate receptors on the postsynaptic MSN in the nucleus accumbens. The receptors open and allow positive ions (Na+ and Ca2+) to enter the cell. In the presence of low synaptic pH, protons bind to ASIC1A, allowing further influx of positive ions into the cell.
Panel E: As positive ions build up within the cell, they induce a strong electric current. The current and buildup of Ca2+ initiates a cascade of intracellular events leading to structural changes that blunt the cell’s responsiveness to cocaine reward and cocaine-associated memories.
- Text Description of Figure 2
-
Panel A: A nonstimulated presynaptic neuron (left) contains vesicles that store both glutamate and protons. The presynaptic neuron is adjacent to a medium spiny neuron (MSN; right) that possesses two receptor types: glutamate and an acid-sensing ion channel, ASIC1A. The glutamate and ASIC1A receptors on the postsynaptic MSN are in a non-activated (closed) state. The synapse between the neurons contains positive (Ca2+, Na+) and negative ions (Cl-).
Panel B: The presynaptic neuron (left) is activated. A vesicle fuses with the presynaptic cell membrane, releasing its contents (glutamate and protons) into the synapse between the presynaptic neuron and postsynaptic MSN (right).
Panel C: The accumulation of protons in the synapse causes the pH to decrease, making the synapse more acidic.
Panel D: Glutamate and protons in the synapse bind to glutamate and ASIC1A receptors, respectively, on the postsynaptic MSN. The receptors are open, allowing the positive ions (Ca2+, Na+) to enter the cell. The positive charges accumulate within the postsynaptic MSN.
Panel E: The accumulation of positive charges within the postsynaptic MSN produces a strong electric current that travels down the cell. Concurrently, the build-up of Ca2+ within the postsynaptic MSN stimulates Ca2+-dependent mechanisms within this neuron.
A New Treatment Strategy?
Although many of the Iowa researchers’ findings were surprising in light of earlier studies on ASICs, the surprise appears to be a happy one. “Sometimes when a hypothesis in not borne out as expected by the experiments, science is led to novel investigations that were impossible to foresee,” says Dr. Thomas Radman, Program Officer and Health Scientist Administrator at NIDA’s Integrative Neuroscience Branch. “In this case, findings regarding the importance of ASIC-dependent, currents in the NAc may yield new therapeutic strategies for the treatment of addiction.”
According to Dr. Wemmie, means for implementing such strategies may already be at hand. “Brain pH can be altered in a variety of ways,” Dr. Wemmie says. “For example, medications have been developed that block carbonic anhydrases, which are enzymes that change the pH in the body’s tissues. Such medications might be deployed in a new approach to treat drug addiction by increasing ASIC1A activity to reduce drugs’ addictive effects.”
This work was supported by NIH grant DA034684.
Source:
Kreple, C.J.; Lu, Y.; Taugher, R.J. et al. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nature Neuroscience 17(8):1083-1091, 2014. Full text
