This study found that:
- A single nucleotide polymorphism (SNP) in the messenger RNA of the µ-opioid receptor gene was associated with patients’ responses to methadone treatment for opioid dependence.
- The association may occur because one variant of the SNP produces fewer µ-opioid receptors than the alternate form.
Methadone helps people remain abstinent from opioids by reducing withdrawal symptoms and craving for the drugs. However, the medication doesn’t work equally well for all patients. New research indicates that a genetic difference may be one reason why.
Drs. Richard C. Crist and Glenn A. Doyle from the Center for Neurobiology and Behavior at the University of Pennsylvania School of Medicine and colleagues focused on the OPRM1 gene as a locus where variation in the sequence of DNA might affect responses to methadone and buprenorphine. OPRM1 encodes the μ-opioid receptor, where all opioids, licit and illicit, including the two opioid agonist medications, exert their effects on the brain. If a variant in OPRM1 were to suppress the gene’s expression, reducing the quantity of receptors available, those effects might be reduced accordingly.
Drs. Crist and Doyle and colleagues hypothesized that if such an OPRM1 variant existed, its suppressive effect might occur in the messenger RNA (MOR-1) transcribed from the gene. The researchers observed that MOR-1 contains an unusually long 3’ untranslated region (3’ UTR) (see Figure 1), and other research had linked lengthy 3’ UTRs in other genes to expression-suppressing mechanisms.
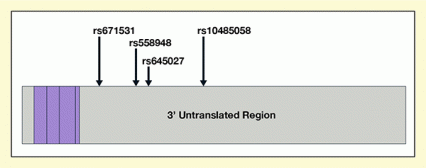 Figure 1. Schematic Representation of the mRNA (MOR-1) Transcribed From the OPRM-1 Gene The MOR-1 regions that translate into the μ-receptor protein are shown in purple; the untranslated region are in gray. The arrows indicate the locations of the four SNPs in the 3’ untranslated region of the mRNA that were analyzed in the studies. rs10485058 was associated with differing responses to methadone, but not to buprenorphine.
Figure 1. Schematic Representation of the mRNA (MOR-1) Transcribed From the OPRM-1 Gene The MOR-1 regions that translate into the μ-receptor protein are shown in purple; the untranslated region are in gray. The arrows indicate the locations of the four SNPs in the 3’ untranslated region of the mRNA that were analyzed in the studies. rs10485058 was associated with differing responses to methadone, but not to buprenorphine.
- Text Description of Figure 1
-
The figure shows a graphic representation of the MOR-1 mRNA that encodes the μ-opioid receptor. The part of the mRNA that encodes the actual receptor protein is represented by the purple section. The noncoding untranslated regions are represented by the gray sections. The long gray section at the right represents the 3’ untranslated region. Arrows indicate the locations of four sequence variants (single-nucleotide polymorphisms, or SNPs) in the 3’ untranslated region, namely, from left to right, rs671531, rs558948, rs645027, and rs10485058.
Contrasting Treatment Responses
The researchers analyzed DNA and treatment-response data from patients who participated in the Starting Treatment with Agonist Replacement Therapy (START) clinical trial. START randomly assigned first-time seekers of treatment for opioid dependence to receive maintenance treatment with either methadone or buprenorphine for 24 weeks at federally licensed opioid treatment programs.
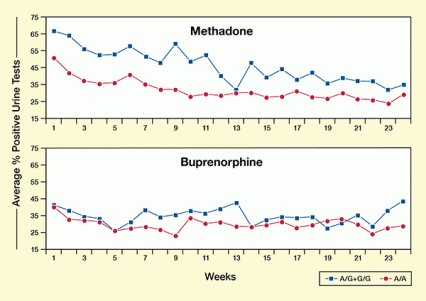 Figure 2. European American Patients Carrying the A/A Genotype at SNP rs10485058 Were Less Likely To Experience Treatment Failure After Methadone Therapy The figure compares the average percentage of opioid-positive urine samples in patients carrying at least one G allele of SNP 10485058 (blue line) and those carrying only the A allele (red line) during 24 weeks of opioid replacement therapy with methadone or buprenorphine. (top) In methadone-treated patients, treatment failure as determined by detection of opioids other than methadone in weekly urinalysis data was significantly lower in patients with the A/A OPRM1 genotype than in those with an A/G or G/G genotype. (bottom) OPRM1 genotype did not predict treatment failure for patients in the buprenorphine group.
Figure 2. European American Patients Carrying the A/A Genotype at SNP rs10485058 Were Less Likely To Experience Treatment Failure After Methadone Therapy The figure compares the average percentage of opioid-positive urine samples in patients carrying at least one G allele of SNP 10485058 (blue line) and those carrying only the A allele (red line) during 24 weeks of opioid replacement therapy with methadone or buprenorphine. (top) In methadone-treated patients, treatment failure as determined by detection of opioids other than methadone in weekly urinalysis data was significantly lower in patients with the A/A OPRM1 genotype than in those with an A/G or G/G genotype. (bottom) OPRM1 genotype did not predict treatment failure for patients in the buprenorphine group.
- Text Description of Figure 2
-
The two graphs show the likelihood of treatment failure in patients treated for opioid dependence with methadone (top panel) or buprenorphine (bottom panel) who carry different variants, or alleles, at SNP rs10485058. The horizontal x-axis shows the treatment duration in weeks from week 1 to week 24. The vertical y-axis shows the average percentage of urine samples that tested positive for opioids at each timepoint. The blue curves with measurement points indicated as squares represent the results for patients who carry at least one G allele of rs10485058. The red curves with measurement points indicated as circles represent the results for patients who carry only the A allele. In the methadone panel, the measurement points for the red curve are clearly below the measurement points of the blue curve at all time points, indicating that patients with only A alleles at rs10485058 were significantly less likely to submit positive urine samples and thus less likely to experience treatment failure. In the buprenorphine panel, the measurement points for the red curve are for most time points close to or even above those of the blue curve, indicating that no significant difference exists between the two groups and that OPRM1 genotype did not predict treatment failure in these patients.
The analysis turned up one SNP (rs10485058) in the START participants’ MOR-1 3’ UTR that appeared to affect how they fared in methadone maintenance (see Figure 2). Methadone-maintained patients of European descent who had at least one copy of the guanine variant of rs10485058 produced more illicit-opioid-positive urine samples during the trial than patients who had only the adenine variant (see Table). Three other SNPs in the patients’ MOR-1 3’UTR (rs671531, rs558948, rs645027) were not associated with methadone treatment response. No SNP was associated with responses to buprenorphine.
A second analysis, this one of data from 1,215 Australians of European descent who were treated with opioid agonist therapy in the Comorbidity and Trauma Study (CTS), reinforced these findings. CTS participants who had at least one copy of the guanine variant of rs10485058 had significantly higher rates of self-reported relapse than those with only the adenine variant.
| SNP combination # | SNP variants in the combination | Treatment failure with methadone (P-value) | Treatment failure with buprenorphine (P-value) | |||
|---|---|---|---|---|---|---|
| rs671531 | rs558948 | rs645027 | rs10485058 | |||
| 1 | G | C | A | A | 0.30 | 0.34 |
| 2 | A | T | A | A | 0.20 | 0.51 |
| 3 | G | C | A | G | 0.0025 | 0.27 |
| 4 | G | C | G | A | 0.34 | 0.33 |
| 5 | A | C | A | A | 0.35 | 0.81 |
A Receptor Disadvantage
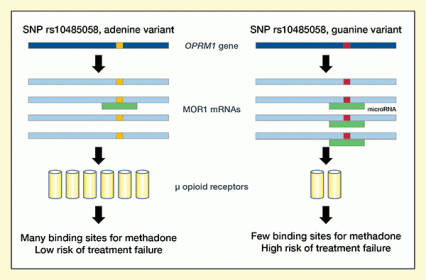 Figure 3. Proposed Mechanism for Higher Treatment Failure With the SNP rs10485058 Guanine Variant MOR-1 mRNAs created from the OPRM1 gene with the adenine variant at SNP rs10485058 are less likely to bind with microRNAs than MOR-1 mRNAs created from the OPRM1 gene with the guanine variant. As a result, more μ-opioid receptors can be produced from the MOR-1 mRNAs with the adenine variant, offering more binding sites for methadone and thus greater efficacy and reduced risk of treatment failure.
Figure 3. Proposed Mechanism for Higher Treatment Failure With the SNP rs10485058 Guanine Variant MOR-1 mRNAs created from the OPRM1 gene with the adenine variant at SNP rs10485058 are less likely to bind with microRNAs than MOR-1 mRNAs created from the OPRM1 gene with the guanine variant. As a result, more μ-opioid receptors can be produced from the MOR-1 mRNAs with the adenine variant, offering more binding sites for methadone and thus greater efficacy and reduced risk of treatment failure.
- Text Description of Figure 3
-
The figure shows the proposed mechanism through which a person’s specific variant at the SNP rs10485058 in the 3’ untranslated region of the MOR-1 mRNAs can influence risk of treatment failure with methadone. The dark blue bar at the top indicates the OPRM1 gene that encodes the μ-opioid receptor. The light blue bars underneath represent the MOR-1 mRNA molecules created from the OPRM1 gene. The orange squares within the bars on the left side indicate the adenine variant of rs10485058, and the red squares in the bars on the right side indicate the guanine variant. The green rectangles represent inhibitory microRNA molecules that can bind to the MOR-1 mRNA and prevent its translation into μ-opioid receptor molecules, which are indicated by yellow cylinders.
A microRNA molecule is indicated only next to one of the four MOR-1 mRNAs on the left side with the adenine rs10485058 variant, but next to three of the four MOR-1 mRNAs on the right side with the guanine variant. This indicates that the guanine variant is more likely to bind the microRNA than the adenine variant and that as a result fewer μ-opioid receptor molecules can be produced from the MOR-1 mRNAs with a guanine variant than from the MOR-1 mRNAs with an adenine variant. Fewer receptors mean fewer potential binding sites where methadone can exert its effects and, consequently, a higher risk of treatment failure.
Drs. Crist and Doyle and colleagues next addressed why patients with the guanine variant of rs10485058 might benefit less from methadone therapy than those with the adenine variant. One possible explanation was that the guanine variant produces fewer μ-opioid receptors than the adenine variant (see Figure 3). If that were the case, methadone and other opioids would have fewer sites to attach to and exert their therapeutic effects on cells.
Prior research had identified a mechanism that produces such differences and pertains especially to genes whose messenger RNA, like that of OPRM1, contains lengthy 3’ UTRs. Such messenger RNA can contain sequences that bind strongly with very short strips of RNA called microRNAs. When protein complexes containing microRNAs attach to messenger RNA, they impede the production of protein from the messenger RNA.
Dr. Crist, Dr. Doyle, and colleagues hypothesized that the guanine variant of rs10485058 binds to microRNAs more readily than the adenine variant, and hence produces fewer μ-opioid receptors. To test the hypothesis, the researchers cloned the two rs10485058 variants into the 3’ UTR of the luciferase gene, which produces the light-generating protein found in fireflies. Cells were cultured with these cloned genes and molecules that mimic microRNAs. A readout of the light emitted from the cultures showed that the microRNA mimic reduced the genetic activity of the guanine rs10485058 RNA more than it did that of the adenine variant.
The researchers hypothesized that differences between methadone and buprenorphine might explain their finding that variation in rs10485058 affected patients’ responses to methadone, but not to buprenorphine. Reduced μ-opioid receptor availability might be expected to inhibit methadone’s effects more than those of buprenorphine because methadone exerts its effects entirely through that receptor, while buprenorphine does so only partially.
Drs. Crist and Doyle and colleagues consider their results to be promising and to warrant additional replication in other populations. If such studies support the observed pharmacogenetic effect of rs10485058 on methadone treatment outcomes, physicians may have a new tool to guide treatment decisions for optimal patient outcomes.
The study was supported by NIH grant number DA036751.
Source:
Crist, R.C.; Doyle, G.A.; Nelson, E.C. et al. A polymorphism in the OPRM1 3'-untranslated region is associated with methadone efficacy in treating opioid dependence. Pharmacogenomics Journal doi: 10.1038/tpj.2016.89, 2016. Full text
