Researchers found that:
- N-acetylcysteine did not help adults reduce their cannabis use, despite having been effective for adolescents in a previous trial.
- If adults are able to benefit from the medication, they will likely require a different treatment regimen than adolescents.
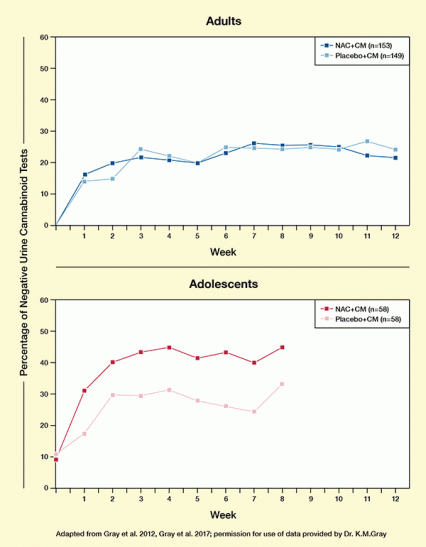 Figure. N-Acetylcysteine (NAC) Promotes Abstinence From Cannabis in Adolescents but not in Adults In two separate clinical trials, adults (top) or adolescents (bottom) with cannabis use disorder received either 1,200 mg NAC twice a day or placebo. All participants also received a contingency management (CM) intervention. NAC-treated and placebo-treated adults submitted similar proportions of cannabis-free urine samples. In contrast, NAC-treated adolescents submitted a significantly higher proportion of cannabis-free urine samples than did placebo-treated adolescents.
Figure. N-Acetylcysteine (NAC) Promotes Abstinence From Cannabis in Adolescents but not in Adults In two separate clinical trials, adults (top) or adolescents (bottom) with cannabis use disorder received either 1,200 mg NAC twice a day or placebo. All participants also received a contingency management (CM) intervention. NAC-treated and placebo-treated adults submitted similar proportions of cannabis-free urine samples. In contrast, NAC-treated adolescents submitted a significantly higher proportion of cannabis-free urine samples than did placebo-treated adolescents.
- Text Description of Figure
-
The figure shows two line graphs illustrating that N-acetylcysteine (NAC) promotes abstinence from cannabis in adolescents but not in adults. The top panel shows the data obtained in a study of adults with cannabis use disorder; the bottom panel shows the data obtained in a separate study of adolescents with cannabis use disorder. In both panels, the horizontal (x) axis shows the treatment duration in weeks from 0 to 12 weeks. In both panels, the vertical (y) axis shows the percentage of negative urine cannabinoid tests among the samples submitted at each time point from 0 to 60 percent. Both panels show a darker curve for participants who were treated with 1,200 mg NAC twice per day and a lighter curve for participants who received a placebo. Among both adults and adolescents, all participants also received a contingency management (CM) intervention.
In the top panel for adult participants, the percentage of negative urine samples is 0 at the start of the study, increases for both groups to about 15 percent by week 1 and 20 percent by week 2, and remains stable between 20 percent and 25 percent throughout the 12-week study period. The curves for participants with and without NAC treatment are close together for all time points, indicating that NAC has no treatment effect.
In the bottom panel for adolescent participants, the percentage of negative urine samples is 10 percent at study begin for both groups. For those treated with NAC, the percentage increases to about 32 percent by week 1, 40 percent by week 2, and remains between 40 percent and 45 percent for the remainder of the 8-week study period. For adolescents treated with placebo, however, the percentage of negative urine samples increases to only about 15 percent by week 1, 30 percent by week 2, and then fluctuates between 25 percent and 35 percent for the rest of the study period. This indicates that NAC can improve treatment success in adolescents.
In a 12-week trial with more than 300 cannabis-dependent adults, those treated with N-acetylcysteine (NAC) submitted no more cannabis-free urine samples (22.3 percent) than those given a placebo (22.4 percent). The disappointing result contrasts with a previous trial in which the medication helped cannabis-dependent adolescents reduce their cannabis use (see Figure).
Researchers are seeking explanations for the contrasting results. One possibility, says Dr. Kevin Gray of the Medical University of South Carolina, who led both trials, is that the results reflect differences between adolescents and adults in cannabis exposure. At the time of their entry into their respective trials, the adults had used the drug for more years than the adolescents and were using it in greater amounts and on more days of the month. Dr. Gray says, “We speculate that with more entrenched, chronic, and high-dose cannabis use, the ‘bar’ necessary for treatment success may be higher.”
Dr. Udi Ghitza of NIDA’s Center for Clinical Trials Network concurs. Noting that participants in both trials received the same dosage of NAC (1,200 mg twice daily), he says, “There may be a need for dose adjustment based on chronicity and frequency of cannabis use.” Dr. Ghitza hypothesizes that more potent NAC derivatives, such as N-acetylcysteine amide, might yield higher rates of cannabis abstinence.
Dr. Gray suggests that psychosocial factors might underlie adolescents’ superior outcomes in the two trials. He explains, “Adolescents may have more active psychosocial motivators and supports for cannabis cessation, such as parents’ concerns, academic performance, and school rules, whereas adults may have more established psychosocial patterns reinforcing their longstanding cannabis use.”
Psychosocial influences may have enhanced adolescents’ responses to NAC directly and also indirectly, by enabling them to benefit from a contingency management (CM) intervention that was delivered in both trials. Dr. Gray explains, “CM may act as an abstinence inducer, and NAC may complement this effect by preventing relapse. If someone is insufficiently responsive to CM, they may not achieve the initial abstinence that may be necessary for NAC to yield its effects.”
Although unsuccessful overall, the adult trial did produce a positive finding. Dr. Gray comments, “Although our second trial did not establish NAC’s efficacy for adults, it lent further support for the medication’s role as a complement to CM in treating cannabis use disorder in adolescents up to age 21. Participants ages 18 to 21 in the adult trial appeared to respond more favorably to NAC than placebo, in a similar magnitude to that seen in the adolescent trial.”
Currently, Dr. Gray and colleagues are conducting a trial to determine whether NAC requires concomitant CM to be effective for adolescents. Participants are receiving either NAC alone (without CM) or placebo. If the two groups’ abstinence rates turn out not to be significantly different, the result will suggest that CM is a necessary platform treatment for adolescents with cannabis use disorder.
Noting that NAC has shown evidence, in vitro and in animals, of efficacy for treating a variety of drug use disorders, Dr. Gray says, “We’re ultimately hoping to determine the best real-world clinical applications of this medication. Our team and others are investigating NAC across a number of substance use disorders and psychiatric disorders in various age groups, and working with preclinical researchers and neuroimagers in an effort to cross-translate mechanistic and clinical findings.”
This research was supported by NIH grants DA026777, DA013727, DA015831, DA020024, DA013714, DA013732, DA013045, and DA042114.
Sources:
Gray, K.M., Carpenter, M.J., Baker, N.L., et al. A double-blind randomized control trial of N-acetylcysteine in cannabis-dependent adolescents. American Journal of Psychiatry 169(8):805-812, 2012.
Gray, K.M., Sonne, S.C., McClure, E.A., et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug and Alcohol Dependence 177:249-257, 2017.
