This study:
- Demonstrated how tetrahydrocannabinol (THC) produces aversive effects in mice.
- Suggests a mechanism to explain why people experience rewarding, aversive, or mixed effects from marijuana.
Not everyone who tries marijuana likes it. The drug makes some people depressed and anxious. Rodents are averse to marijuana’s main psychoactive ingredient, THC, and will not self-administer it.
What accounts for these negative reactions? New research by Dr. Zheng-Xiong Xi and colleagues in NIDA’s Intramural Research Program (IRP) and the Beijing Institute of Pharmacology and Toxicology shows that:
- THC inhibits glutamate-releasing (glutamatergic) neurons in the brain’s ventral tegmental area (VTA), a key reward-processing region.
- In mice, inhibiting these neurons reduces behaviors that indicate well-being and pleasure, and promotes aversive responses to places that animals associate with the inhibition.
The new research suggests that THC’s effects on mood derive from its inhibition of two types of neurons that regulate how much dopamine is released into the brain’s reward center. Whether the drug is experienced as rewarding or aversive depends in large part on which of the two neuron types is inhibited more.
Cannabis Aversion
The IRP–Beijing researchers began by establishing that VTA glutamatergic neurons express cannabinoid type-1 (CB1) receptors. When these receptors are present in a neuron, THC can attach to them and inhibit the neuron’s activity. In these experiments, the researchers used a technique called in situ hybridization, which seeks out and labels specific genetic material, to reveal that VTA glutamatergic neurons contain CB1 messenger RNA (mRNA). The presence of this mRNA indicates that the neurons express CB1, because the mRNA is an intermediate product in the production pathway from the CB1 gene to the completed receptor.
To show that THC inhibition of VTA glutamatergic neurons has an aversive effect, the researchers conducted experiments with two groups of mice. One group were normal (wild-type), and the other group were mice that were genetically engineered to lack CB1 receptors on their VTA glutamatergic neurons.
In one experiment, the researchers placed mice in a dual-chamber environment, then repeatedly exposed them to THC in one of the chambers. Once the mice learned to associate that chamber with the sensations imparted by THC, both groups spent markedly less time there than in the other chamber, indicating that they felt aversion to the drug-induced sensations (see Figure 1). However, the normal mice, in whom THC inhibited VTA glutamatergic neurons, shunned the chamber more than the mice whose glutamatergic neurons lacked CB1. The difference indicates that THC inhibition of VTA glutamatergic neurons intensified the aversive response of the normal mice.
In a confirmatory experiment, the researchers exposed mice to THC and observed the effect on their locomotor activity. An animal’s locomotor response to a drug—how much more or less it ambulates after exposure to the drug—reflects how rewarding or unpleasant it finds the drug. THC exposure sharply reduced locomotion among normal mice, indicating a negative response, but did not significantly alter locomotion among their genetically engineered counterparts. These results further indicate that THC causes aversive effects in rodents by activating CB1 receptors on VTA glutamatergic neurons.
Pathway to the Reward Center
The IRP–Beijing researchers had established that THC inhibition of VTA glutamatergic neurons underlies the drug’s aversive effect. They next turned to the question, how?
They hypothesized that VTA glutamatergic neurons promote reward-system activity that supports positive mood. THC inhibition of these neurons consequently has the opposite effect. It reduces reward-system activity and lowers mood, so much that it ultimately produces aversion.
To test their hypothesis, the researchers devised a new experimental technique, called optical intracranial self-stimulation (oICSS). In oICSS, mice press a lever to activate an optical fiber that directly and selectively stimulates their VTA glutamatergic neurons.
In two oICSS experiments, the researchers linked:
- VTA glutamatergic stimulation to reward: The researchers observed how mice changed their oICSS behavior as the amount of stimulation delivered with each lever press increased. The higher the stimulation, the more frequently the mice pressed the lever, indicating that, to them, the more VTA glutamatergic activity, the better.
- THC inhibition of VTA glutamatergic activity to reduced reward: The researchers observed the oICSS behavior of the mice after exposure to THC. Normal mice, in whom THC inhibits VTA glutamatergic neurons, reduced the frequency of their lever pressing, indicating diminishing reward, proportionally as the researchers increased the THC dose. In contrast, mice from the researchers’ genetically modified line, in whom THC does not inhibit VTA glutamatergic neurons (since CB1 is deleted), pressed the lever just as often regardless of the THC dose.
A further experiment elucidated the pathway that leads from VTA glutamatergic stimulation to rewarding experience. The researchers posited that glutamatergic neurons stimulate dopaminergic neurons to release the mood-elevating neurotransmitter dopamine into the brain’s reward center. As evidence for this pathway, they showed that treating animals with a compound that prevents dopamine from activating its receptors in the reward center reduced the rewarding effect of oicss VTA stimulation.
What About People?
Many people, of course, use marijuana recreationally. What accounts for the difference between human and rodent responses to the drug?
The IRP–Beijing study, together with other evidence, suggests that THC will be experienced as pleasurable or otherwise depending largely on its net effect on two sets of neurons (see Figure 2). In addition to glutamatergic neurons, the VTA is also home to neurons that release the neurotransmitter gamma-aminobutyric acid (GABA). Previous studies have demonstrated that these two types of neurons exert opposite effects on VTA dopamine-releasing neurons. Whereas glutamatergic neurons stimulate the dopaminergic neurons to release dopamine into the brain’s reward center, GABA-ergic neurons inhibit them. Consequently, THC inhibition of VTA glutamate neurons indirectly reduces dopamine activity in the reward center, leading to aversion, and THC inhibition of GABA-ergic neurons increases dopamine activity, producing euphoria.
In the rodent VTA, the researchers note, glutamatergic neurons produce more CB1 mRNA, and thus more CB1 receptors, than do GABA-ergic neurons. Hence, when the rodent VTA is exposed to THC, the drug’s inhibition of CB1 in glutamatergic neurons predominates, producing primarily aversive effects. In the human VTA, in contrast, CB1 levels may be more similar in glutamatergic and GABA-ergic neurons. As a result, when a person is exposed to THC, the experience can be rewarding, aversive, or neutral.
The study was supported by the NIDA Intramural Research Program.
- Text Description of Figure 1
-
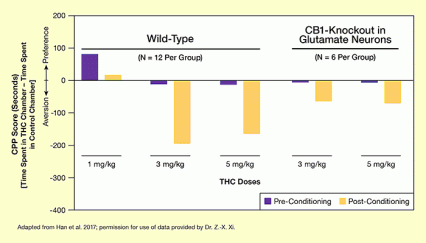
The bar chart illustrates the aversive effects of different doses of THC in normal (wild-type) mice and in genetically engineered mice in which the CB1 receptor was specifically inactivated in VTA glutamatergic neurons (CB1-knockout). The effects of THC were determined using a conditioned place preference (CPP) score, which is plotted on the vertical (y) axis. This score is measured in seconds and is calculated as the time the animals spent in a THC-associated chamber minus the time the animals spent in a control chamber when allowed to freely move between chambers in a three-chamber experimental setup. CPP score values above the horizontal line representing 0 indicate a preference for the drug, whereas CPP scores below 0 indicate aversion to the drug.
The two groups of animals were tested before and after repeated exposure to different THC doses. The CPP score determined before the beginning of THC administration (pre-conditioning) is indicated by purple bars, and the score determined after repeated THC administration (post-conditioning) is indicated by yellow bars. For both animal groups and all THC doses, THC had an aversive effect as indicated by lower post-conditioning than pre-conditioning CPP scores. For wild-type animals, repeated administration of 1 mg/kg THC led to a decline in CPP score from about +90 seconds to about +10 seconds, administration of 3 mg/kg THC led to a decline in CPP score from about -5 seconds to about -200 seconds, and administration of 5 mg/kg THC led to a decline in THC score from about -10 seconds to about -170 seconds. For the CB1-knockout animals, administration of 3 mg/kg THC led to a decline in CPP score from about -3 seconds to about -70 seconds, and administration of 5 mg/kg THC to a decline in CPP score from about -3 seconds to about -80 seconds. Thus, the aversive effects induced by THC were lower in the CB1-knockout animals than in the wild-type animals.
- Text Description of Figure 2
-
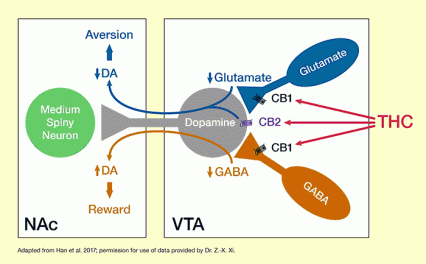
The figure illustrates the proposed mechanism through which THC exerts rewarding and aversive effects. The two white boxes represent two brain areas: the ventral tegmental area (VTA) on the right and the nucleus accumbens (NAc) on the left. The VTA contains neurons releasing the neurotransmitter glutamate (shown in dark blue), neurons releasing the neurotransmitter gamma-aminobutyric acid (GABA) (shown in brown), and neurons releasing the neurotransmitter dopamine (DA), which extend to the NAc (shown in gray). The glutamate and GABA released from these cells act on the dopamine-releasing cell, with glutamate increasing and GABA reducing dopamine release in the NAc. The dopamine then acts on medium spiny neurons in the NAc (shown in green).
The glutamate-releasing and GABA-releasing neurons carry CB1 receptors (indicated by black symbols), and the dopamine-releasing neuron carries a CB2 receptor (indicated by purple symbol). THC can bind to the CB1 and CB2 receptors, indicated by the red arrows. Binding of THC to CB1 receptors reduces glutamate and GABA release from the respective cells. As indicated by the blue arrow reaching from the VTA to the NAc, reduced glutamate release in the VTA reduces dopamine release in the NAc. As indicated by the short and wide blue arrow, this leads to an increase in aversion. Conversely, as indicated by the brown arrow reaching from the VTA to the NAc, reduced GABA release in the VTA leads to increased dopamine release in the NAc. As indicated by the short and wide brown arrow, increased dopamine levels, in turn, lead to increased reward. THC binding to the CB2 receptor on the dopamine-releasing neuron in the VTA also leads to reduced dopamine release and, consequently, increased aversion. The balance of THCs effects on glutamate-releasing and GABA-releasing cells in the VTA determines whether the user experiences mainly the rewarding or aversive effects of THC.
Source:
- Han, X., He, Y., Bi, G.H., et al. CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies Δ9-tetrahydrocannabinol (Δ9-THC)-induced aversive effects in mice. Sci Rep 7(1):12315, 2017.
About the Researchers
Over the past 15 years, Dr. Xi and colleagues at NIDA have investigated the circumstances under which cannabis is aversive, how cannabis produces aversive effects, and whether these aversive effects have therapeutic potential. In addition to the above findings on CB1, Dr. Xi and his colleagues also found a new cannabinoid receptor—the CB2 receptor—in the brain, particularly in midbrain dopamine neurons. Since brain dopamine is a critical neural substrate mediating drug reward, and cannabis activation of CB2 receptors can inhibit midbrain dopamine neurons, he has recently proposed that cannabis’ actions on brain CB2 receptors may also in part contribute to its aversive effects. The Xi laboratory is also exploring the roles of CB1 receptors found in midbrain GABA-ergic neurons, a subpopulation of dopamine neurons, and astrocyte or microglial glial cells, in cannabis reward and aversion.